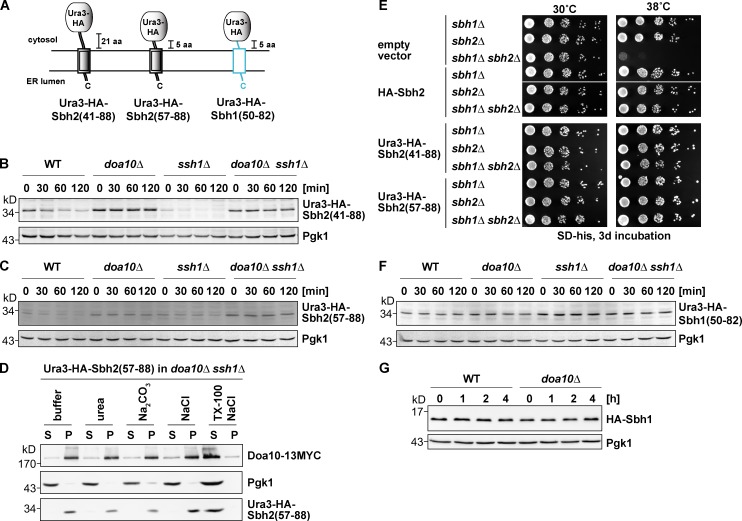
Figure 3.
Mapping of the degron within Sbh2. Analysis of Ura3-HA-Sbh2 fusion proteins. (A) Schematic depiction of Ura3-HA-Sbh2 and Ura3-HA-Sbh1 fusion proteins used in this study. Sequence regions derived from Sbh1 are in light blue, and Sbh2 derived sequences are depicted as double black lines. (B) The last 48 residues of Sbh2 promote degradation of a stable protein. Degradation of ectopically expressed Ura3-HA-Sbh2(aa 41–88) (low-copy plasmid; MET25 promoter) in WT, doa10Δ, ssh1Δ, and doa10Δ ssh1Δ cells. chx chase was performed as in Fig. 1 B. (C) The last 32 residues of Sbh2 promote degradation of a stable protein. Degradation of ectopically expressed Ura3-HA-Sbh2(aa 57–88) as in B. (D) Ura3-HA-Sbh2(aa 57–88) is an integral membrane protein in ssh1Δ cells. Subcellular fractionation of doa10Δ ssh1Δ cells expressing Ura3-HA-Sbh2(aa 57–88) as in Fig. 1 E. S, supernatant; P, pellet; TX-100, Triton X-100. (E) Suppression of growth defect of sbh1Δ sbh2Δ cells at a high temperature by HA-Sbh2 and Ura3-HA-Sbh2 fusion proteins. Cells were transformed with an empty vector (p413MET25) or a p413MET25-based plasmid encoding HA-Sbh2 or the indicated Ura3-HA-Sbh2 protein. Serial dilutions (sixfold) of cultures were spotted onto plates, and plates were incubated as indicated. Empty vector and HA-Sbh2 lanes are from one plate (one plate for 30°C and a second one for 38°C). Both Ura3-HA-Sbh2(aa 41–88) and Ura3-HA-Sbh2(aa 57–88) lanes are from plates that were incubated parallel to the empty vector and HA-Sbh2 plates. (F) The Ura3-HA-Sbh1(aa 50–82) fusion protein is stable. chx chase with ectopically expressed Ura3-HA-Sbh1(aa 50–82) as in B. (G) Sbh1 is a stable protein. chx chase with ectopically expressed HA-Sbh1 (as in B) in WT and doa10Δ cells.