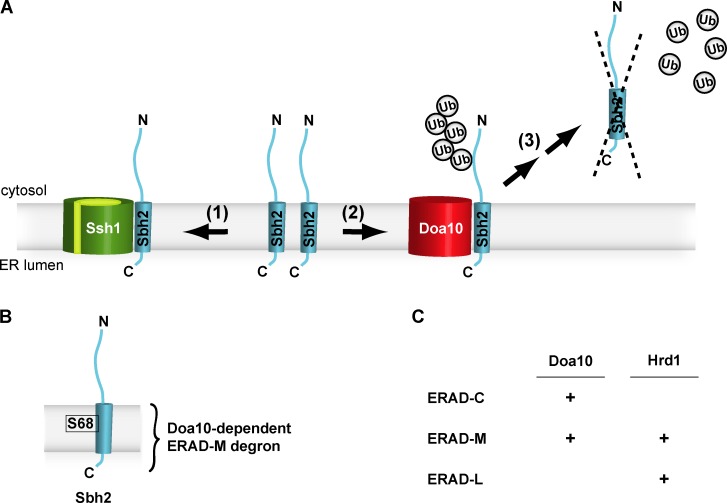
Figure 6.
Model for Sbh2 quantity control via a Doa10-dependent intramembrane degron. (A) The TA Sbh2 protein associates with the Ssh1 protein in the ER membrane (1). Together with a third integral membrane protein, Sss1 (not depicted), Ssh1, and Sbh2 form the heterotrimeric Ssh1 complex implicated in cotranslational protein translocation in S. cerevisiae (besides the heterotrimeric Sec61 complex). Assembled Sbh2 is stable/protected from degradation. In contrast, unassembled Sbh2 (e.g., in ssh1Δ cells or surplus Sbh2 in WT cells) is readily degraded via a Doa10-dependent ERAD pathway involving ubiquitylation (2), retrotranslocation, and proteasomal degradation of Sbh2 (3). Ub, ubiquitin. (B) Schematic depiction of the Doa10-dependent intramembrane (ERAD-M) degron of Sbh2. The degron encompasses the Sbh2 TM domain and the short ER-luminal domain. The serine residue at position 68 (S68) located within the Sbh2 TM helix is a crucial part of the degron. (C) Assignment of the two major S. cerevisiae ERAD E3 ligases to different ERAD substrate classes. The identification of a Doa10-dependent ERAD-M substrate, Sbh2, allows assigning Doa10 to the ERAD-M pathway.