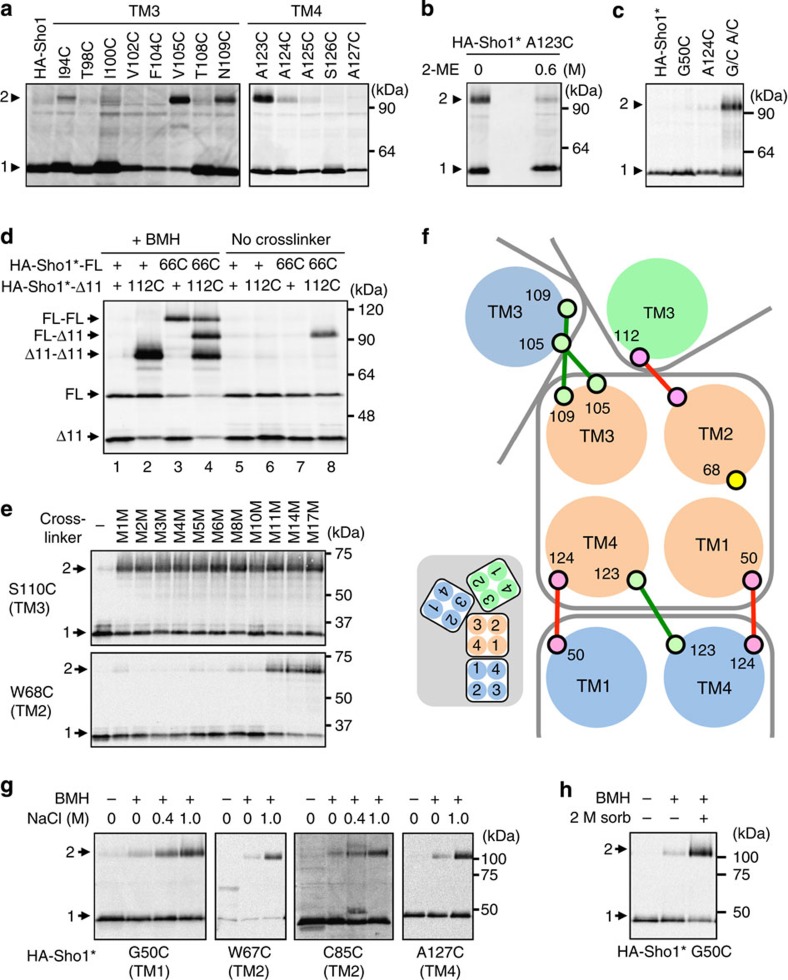
Figure 7. Arrangements of the four TM domains in the Sho1 oligomers and osmostress-induced structural changes in Sho1.
(a) Spontaneous disulfide bond formation by Sho1 single-Cys substitution mutants. Membrane fractions were subjected to SDS–PAGE (without 2-ME) and HA-Sho1 and its mutant derivatives were detected by immunoblotting. (b) Inhibition of disulfide bond formation by the reductant 2-ME. Sample lanes are intentionally separated by two empty lanes. (c) Spontaneous disulfide bond formation by a Sho1 double-Cys substitution mutant. G/C, G50C; A/C, A124C. (d) Spontaneous disulfide bond formation between two Sho1 single-Cys substitution mutants. Membrane fractions were treated with (+) or without (−) BMH and were subjected to SDS–PAGE (without 2-ME). 66C, T66C; 112C, T112C; Sho1Δ11 is Sho1Δ(296–367). (e) Chemical crosslinking by molecular ruler. Single-Cys substitution mutants of HA-Sho1*Δ11 were chemically crosslinked using a set of crosslinkers with different spacer lengths (M1M–M17M). The procedure was the same as in Fig. 6a, except that SDS–PAGE was done under non-reducing conditions. (f) Model of helix packing at the TM1/4 and TM2/3 interfaces (top view from the outside). Large circles represent TM helices and small circles are amino-acid residues. Red and green lines represent spontaneously formed disulfide bonds. Inset is a smaller scale view of the dimeric and trimeric complexes. (g) Osmostress-induced changes in chemical crosslinking of Sho1. KT079 was transformed with expression plasmids for the indicated mutants of HA-Sho1*. HA-Sho1* was induced by 2% galactose for 2 h, and was crosslinked in intact cells with BMH, in the presence or absence of NaCl, as indicated. (h) Effects of sorbitol on Sho1 crosslinking. HA-Sho1* G50C was expressed in KT079 and crosslinked as in a except in the presence (+) and absence (−) of 2 M sorbitol.