Table 1. Clinical and biochemical characteristics of patients with insulinoma, nesidioblastosis, and insulin autoimmune syndrome.
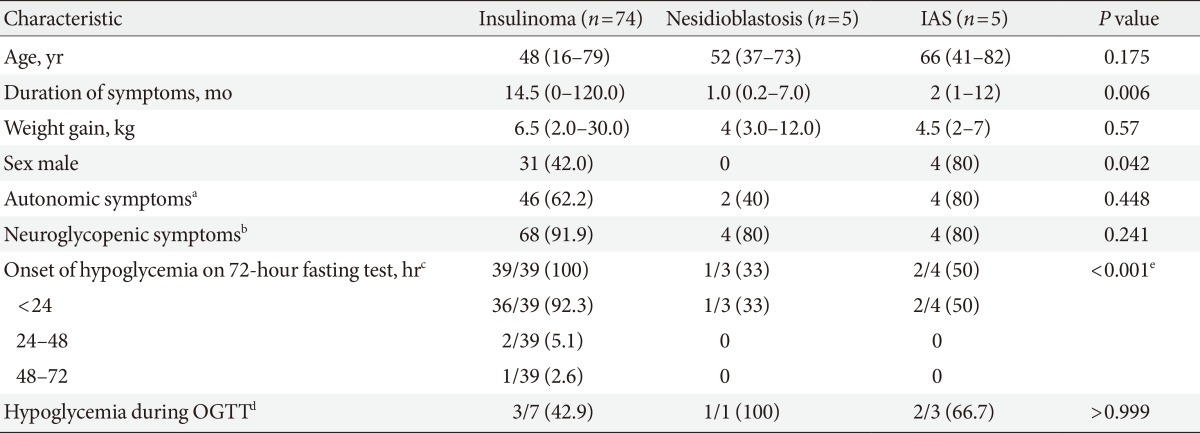
Values are presented as median (range) or number (%).
IAS, insulin autoimmune syndrome; OGTT, oral glucose tolerance test.
aNumber of patients who developed autonomic hypoglycemic symptoms (e.g., sweating, palpitation, anxiety, hunger, nausea, and paresthesia), bNumber of patients who developed neuroglycopenic hypoglycemic symptoms (e.g., loss of consciousness, disorientation, fatigue, seizure, hypersomnia, blurred vision, and change in behavior), cNumber of patients who developed hypoglycemia on 72-hour fasting test/total number of patients who received a 72-hour fasting test, dNumber of patients who developed hypoglycemia during OGTT/total number of patients who underwent OGTT, eStatistically significant development of hypoglycemia on 72-hour fasting test.
