Figure 7.
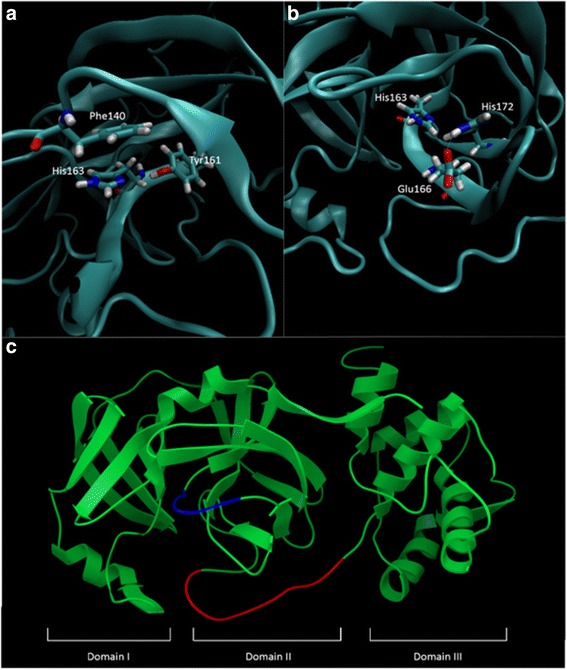
Features present in the homology model of OC43 3CLpro which represent of an active state of the enzyme. (a) Orientation of His163 is essential for substrate binding. This orientation is maintained by stacking interactions with Phe140 and a hydrogen bond with Tyr161. The importance of this bond is questionable as it is not observed in all crystallographic models. (b) Steric interactions between His172 and His163 disrupt the active conformation of His163. To prevent this, His172 is stabilized by a hydrogen bond with Glu166. (c) Maintenance of loop structures of the oxyanion loop (blue) and the loop connecting domain II and III (red) are essential in stabilizing the oxyanion hole. The general three domain structure of all 3CLpro is also depicted.
