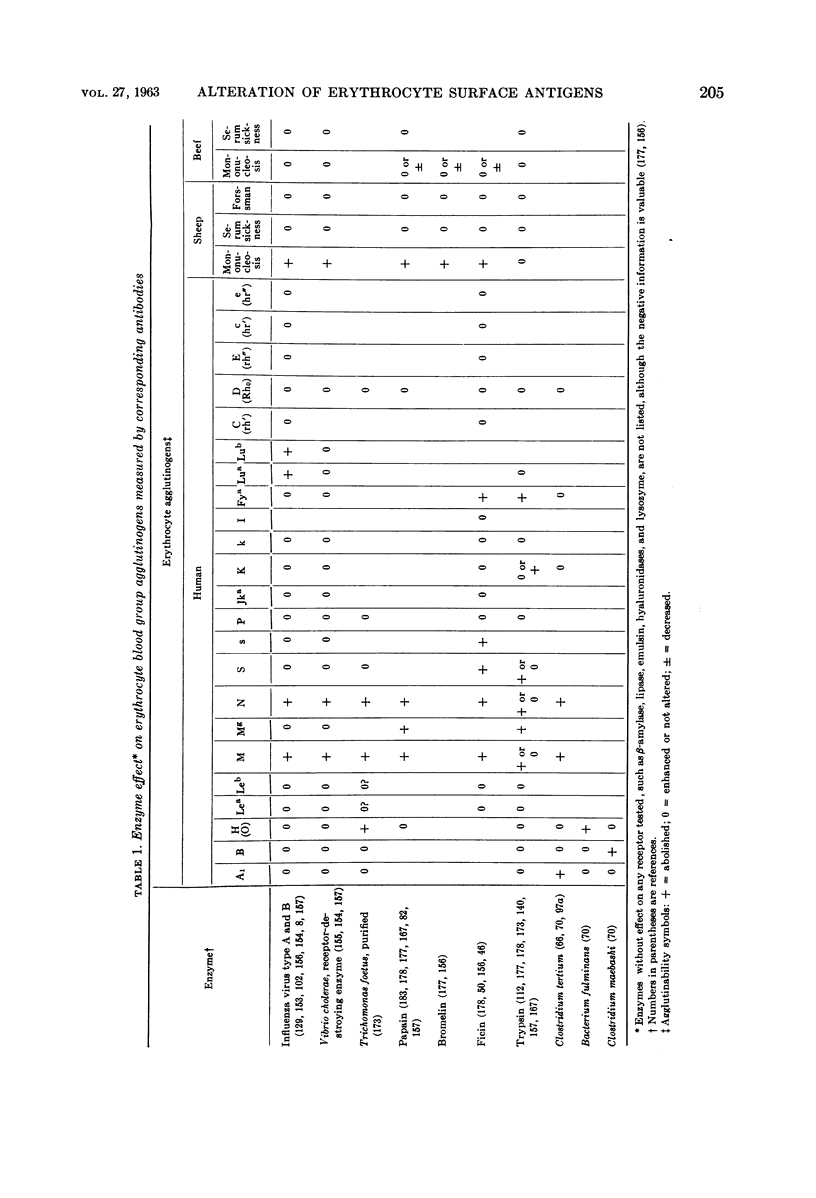
Full text
PDF


































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E., MCFADDEN M., SMITH E. L. Peptidases of erythrocytes. I. Distribution in man and other species. J Biol Chem. 1952 Oct;198(2):663–670. [PubMed] [Google Scholar]
- ALLEN F. H., CORCORAN P. A., ELLIS F. R. Some new observations on the MN system. Vox Sang. 1960 May;5:224–231. doi: 10.1111/j.1423-0410.1960.tb03737.x. [DOI] [PubMed] [Google Scholar]
- ARJONA E., SEGOVIA J. M., ORTEGA A., PERIANES J. Pérdida de la aglutinabilidad de los hematäes por la acción del tanino. Rev Clin Esp. 1953 Jul 15;50(1-2):25–31. [PubMed] [Google Scholar]
- ATWOOD K. C., SCHEINBERG S. L. Somatic variation in human erythrocyte antigens. J Cell Physiol Suppl. 1958 Dec;52(Suppl 1):97–123. doi: 10.1002/jcp.1030520408. [DOI] [PubMed] [Google Scholar]
- AXELROD J., REICHENTHAL J., BRODIE B. B. The direct determination of phosphatidyl ethanolamine and phosphatidyl serine in plasma and red blood cells. J Biol Chem. 1953 Oct;204(2):903–911. [PubMed] [Google Scholar]
- BARRETT J. T. Agglutination and lysis of erythrocytes by lysozyme. Proc Soc Exp Biol Med. 1958 Apr;97(4):794–795. doi: 10.3181/00379727-97-23882. [DOI] [PubMed] [Google Scholar]
- BOURSNELL J. C., COOMBS R. R., RIZK V. Studies with marked antisera; quantitative studies with antisera marked with iodine 131isotope and their corresponding red-cell antigens. Biochem J. 1953 Dec;55(5):745–758. doi: 10.1042/bj0550745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. V. Fixation of bacterial products by erythrocytes in vivo and by Leucocytes. Nature. 1953 Feb 28;171(4348):402–403. doi: 10.1038/171402a0. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADING I. The use of tannic acid to link blood group substances to group O red cells. Aust J Exp Biol Med Sci. 1956 Apr;34(2):157–163. doi: 10.1038/icb.1956.19. [DOI] [PubMed] [Google Scholar]
- BROWN E. A. The adsorption of lipids from the erythrocyte surface by silica and alumina. J Cell Physiol. 1957 Aug;50(1):49–56. doi: 10.1002/jcp.1030500104. [DOI] [PubMed] [Google Scholar]
- Bailey G. H., Raffel S. HEMOLYTIC ANTIBODIES FOR SHEEP AND OX ERYTHROCYTES IN INFECTIOUS MONONUCLEOSIS. J Clin Invest. 1935 Mar;14(2):228–244. doi: 10.1172/JCI100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTWRIGHT G. E., GOLDBERG A., JONES R. S., WILLIAMS C. B., WINTROBE M. M., YANAGITA M. Studies on copper metabolism. XXII. Hemolytic anemia in chickens induced by the administration of copper. J Lab Clin Med. 1956 Sep;48(3):442–453. [PubMed] [Google Scholar]
- CEPPELLINI R., DE GREGORIO M. Emagglutinazione ed emolisi condizionate mediante gli antigeni della Salmonella typhi. Boll Ist Sieroter Milan. 1953 Nov-Dec;32(11-12):429–444. [PubMed] [Google Scholar]
- CHORPENNING F. W., HAYES J. C. Occurrence of the Thomsen-Friedenreich phenomenon in vivo. Vox Sang. 1959 Jul;4:210–224. doi: 10.1111/j.1423-0410.1959.tb05130.x. [DOI] [PubMed] [Google Scholar]
- COFFIN S. F., PICKLES M. M. The ability of trypsin to restore specific agglutinating capacity of erythrocytes treated with periodate. J Immunol. 1953 Oct;71(4):177–182. [PubMed] [Google Scholar]
- COOMBS R. R. A., GLEESON-WHITE M. H., HALL J. G. Factors influencing the agglutinability of red cells. II. The agglutination of bovine red cells previously classified as "inagglutinable" by the building up of an "anti-globulin: globulin lattice" on the sensitized cells. Br J Exp Pathol. 1951 Jun;32(3):195–202. [PMC free article] [PubMed] [Google Scholar]
- COOMBS R. R. A., HOWARD A. N., WILD F. Titration of antisera to soluble proteins on the basis of an agglutination reaction: conjugation of egg albumin and chicken serum globulin to the incomplete Rh antibody and the subsequent use of Rh-positive cells, sensitized by such conjugated incomplete antibodies, to titrate antisera against egg albumin and chicken globulin. Br J Exp Pathol. 1952 Aug;33(4):390–397. [PMC free article] [PubMed] [Google Scholar]
- DAUSSET J., MOULLEC J., BERNARD J. Acquired hemolytic anemia with polyagglutinability of red blood cells due to a new factor present in normal human serum (Anti-Tn). Blood. 1959 Oct;14:1079–1093. [PubMed] [Google Scholar]
- DAVIES D. A., CRUMPTON M. J., MACPHERSON I. A., HUTCHISON A. M. The adsorption of bacterial polysaccharides by erythrocytes. Immunology. 1958 Apr;1(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A. Studies on the mode of action of excess of vitamin A. 5. The effect of vitamin A on the stability of the erythrocyte membrane. Biochem J. 1962 Sep;84:611–621. doi: 10.1042/bj0840611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EJBY-POULSEN P. Haemolytic anaemia produced experimentally in the guinea pig by T-transformation of the erythrocytes in vivo with purified concentrated enzyme. Nature. 1954 Nov 13;174(4437):929–930. doi: 10.1038/174929a0. [DOI] [PubMed] [Google Scholar]
- FASTIER L. B. Studies on haemagglutination with the GDVII strain of murine encephalomyelitis. J Immunol. 1950 Sep;65(3):323–330. [PubMed] [Google Scholar]
- Finland M., Peterson O. L., Allen H. E., Samper B. A., Barnes M. W. COLD AGGLUTININS. II. COLD ISOHEMAGGLUTININS IN PRIMARY ATYPICAL PNEUMONIA OF UNKNOWN ETIOLOGY WITH A NOTE ON THE OCCURRENCE OF HEMOLYTIC ANEMIA IN THESE CASES. J Clin Invest. 1945 Jul;24(4):458–473. doi: 10.1172/JCI101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILES C. M. Survey of uses for ficin in blood group serology. Vox Sang. 1960 Sep;5:467–471. doi: 10.1111/j.1423-0410.1960.tb05225.x. [DOI] [PubMed] [Google Scholar]
- HARLEY J. D., MARGOLIS J. Haemolytic activity of colloidal silica. Nature. 1961 Mar 25;189:1010–1011. doi: 10.1038/1891010a0. [DOI] [PubMed] [Google Scholar]
- HARRIS J. W. Studies on the mechanism of a drug-induced hemolytic anemia. J Lab Clin Med. 1956 May;47(5):760–775. [PubMed] [Google Scholar]
- HILLIER J., HOFFMAN J. F. On the ultrastructure of the plasma membrane as determined by the electron microscope. J Cell Physiol. 1953 Oct;42(2):203–247. doi: 10.1002/jcp.1030420205. [DOI] [PubMed] [Google Scholar]
- HILLIER J., HOFFMAN J. F., PARPART A. K., WOLMAN I. J. Ultrastructure of erythrocyte membranes in thalassemia major and minor. Blood. 1956 Oct;11(10):946–956. [PubMed] [Google Scholar]
- HORNUNG M., BAER H. Effect of tannic acid and adsorbed purified blood group substances on human erythrocytes. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):744–746. doi: 10.3181/00379727-98-24170. [DOI] [PubMed] [Google Scholar]
- HOWE C., MACLENNAN J. D., MANDL I., KABAT E. A. Enzymes of Clostridium tertium: effects on blood group and virus receptor substances. J Bacteriol. 1957 Sep;74(3):365–376. doi: 10.1128/jb.74.3.365-376.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWE C., ROSE H. M., SCHNEIDER L. Enzymic action of influenza virus on human erythrocyte stroma components. Proc Soc Exp Biol Med. 1957 Oct;96(1):88–94. doi: 10.3181/00379727-96-23401. [DOI] [PubMed] [Google Scholar]
- HOYT R. E., ZWICKER H. The role of enzyme in reversible agglutination of red cells. J Immunol. 1953 Nov;71(5):325–330. [PubMed] [Google Scholar]
- HUBINONT P. O., GHYSDAEL P., THYS O. Production of an agglutinating auto-antibody (panagglutinin) active upon tanned erythrocytes in the rabbit. Nature. 1959 Oct 17;184(Suppl 16):1250–1251. doi: 10.1038/1841250a0. [DOI] [PubMed] [Google Scholar]
- HUGHES-JONES N. C., GARDNER B., TELFORD R. The kinetics of the reaction between the blood-group antibody anti-c and erythrocytes. Biochem J. 1962 Dec;85:466–474. doi: 10.1042/bj0850466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., JONES A. R., CASTLE W. B. The destruction of red cells by antibodies in man. I. Observations of the sequestration and lysis of red cells altered by immune mechanisms. J Clin Invest. 1957 Oct;36(10):1428–1459. doi: 10.1172/JCI103542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L. The agglutination and sensitization of red cells by metallic cations: interactions between multivalent metals and the red-cell membrane. Br J Haematol. 1957 Jan;3(1):19–38. doi: 10.1111/j.1365-2141.1957.tb05768.x. [DOI] [PubMed] [Google Scholar]
- KABAT E. A. Structural similarities of methylated D- and L-fucose derivatives as seen in three-dimensional models. Biochem J. 1962 Nov;85:291–293. doi: 10.1042/bj0850291. [DOI] [PubMed] [Google Scholar]
- KATHAN R. H., WINZLER R. J., JOHNSOM C. A. Preparation of an inhibitor of viral hemagglutination from human erythrocytes. J Exp Med. 1961 Jan 1;113:37–45. doi: 10.1084/jem.113.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLENK E., LAUENSTEIN K. Uber die zuckerhaltigen Lipoide der Formbestandteile des menschlichen Blutes. Hoppe Seylers Z Physiol Chem. 1951 Nov;288(4-6):220–228. [PubMed] [Google Scholar]
- KLENK E., STOFFEL W. Zur Kenntnis der Zellreceptoren für das Influenzavirus; über das Vorkommen von Neuraminsäure im Eiweiss des Erythrocytenstromas. Hoppe Seylers Z Physiol Chem. 1956 Feb 7;303(1-2):78–80. [PubMed] [Google Scholar]
- KLENK E., UHLENBRUCK G. Uber ein neuraminsäurehaltiges Mucoproteid aus Rindererythrocytenstroma. Hoppe Seylers Z Physiol Chem. 1958;311(4-6):227–233. [PubMed] [Google Scholar]
- KLENK E., UHLENBRUCK G. [On the isolation of mucoids containing neuraminic acid from human erythrocyte stroma, a contribution to the chemistry of agglutinogens]. Hoppe Seylers Z Physiol Chem. 1960;319:151–160. doi: 10.1515/bchm2.1960.319.1.151. [DOI] [PubMed] [Google Scholar]
- KOPPISCH E., OLIVER-GONZALEZ J. Agglutination of Erythrocytes in vivo in mice after injection of Ascaris extracts, related to immunization with human erythrocytes. Proc Soc Exp Biol Med. 1959 Apr;100(4):827–829. doi: 10.3181/00379727-100-24792. [DOI] [PubMed] [Google Scholar]
- KOSCIELAK J. Reversible inactivation of blood-group A and B substances from red cells. Nature. 1962 May 26;194:751–752. doi: 10.1038/194751a0. [DOI] [PubMed] [Google Scholar]
- LEPOW I. H., RATNOFF O. D., ROSEN F. S., PILLEMER L. Observations on a pro-esterase associated with partially purified first component of human complement (C'1). Proc Soc Exp Biol Med. 1956 May;92(1):32–37. doi: 10.3181/00379727-92-22376. [DOI] [PubMed] [Google Scholar]
- LERNER A. M., CHERRY J. D., FINLAND M. Hemagglutination with reoviruses. Virology. 1963 Jan;19:58–65. doi: 10.1016/0042-6822(63)90024-2. [DOI] [PubMed] [Google Scholar]
- LEVINE P., CELANO M. J., VOS G. H., MORRISON J. The first human blood, ---/---, which lacks the 'D-like' antigen. Nature. 1962 Apr 21;194:304–305. doi: 10.1038/194304a0. [DOI] [PubMed] [Google Scholar]
- LEVINE P., OTTENSOOSER F., CELANO M. J., POLLITZER W. On reactions of plant anti-N with red cells of chimpanzees and other animals. Am J Phys Anthropol. 1955 Mar;13(1):29–36. doi: 10.1002/ajpa.1330130104. [DOI] [PubMed] [Google Scholar]
- LUDERITZ O., WESTPHAL O., SIEVERS K., KROGER E., NETER E., BRAUN O. H. Uber die Fixation von P32-markiertem Lipopolysaccharid (Endotoxin) aus Escherichia coli an menschlichen Erythrocyten. Biochem Z. 1958;330(1):34–46. [PubMed] [Google Scholar]
- MACPHERSON I. A., WILKINSON J. F., SWAIN R. H. The effect of Klebsiella aerogenes and Klebsiella cloacae polysaccharides on haemagglutination by and multiplication of the influenza group of viruses. Br J Exp Pathol. 1953 Dec;34(6):603–615. [PMC free article] [PubMed] [Google Scholar]
- MASOUREDIS S. P. Relationship between RhO(D) genotype and quantity of 1131 anti-RhO(D) bound to red cells. J Clin Invest. 1960 Sep;39:1450–1462. doi: 10.1172/JCI104164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER M. M. Studies on the mechanism of hemolysis by antibody and complement. Prog Allergy. 1958;5:215–270. [PubMed] [Google Scholar]
- MOBERLY M. L., MARINETTI G. V., WITTER R. F., MORGAN H. R. Studies of hemolysis of red blood cells by mumps virus. III. Alterations in lipoproteins of the red blood cell wall. J Exp Med. 1958 Jan 1;107(1):87–94. doi: 10.1084/jem.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOLTEN S. E., CLARK E., GLASSER B. F., KATZ E., MILLER B. S. Blood stream invasion by Newcastle disease virus associated with hemolytic anemia and encephalopathy; report of three cases. Am J Med. 1953 Mar;14(3):294–306. doi: 10.1016/0002-9343(53)90041-2. [DOI] [PubMed] [Google Scholar]
- MORGAN W. T. J., WATKINS W. M. The inactivation of the blood group receptor on the human erythrocyte surface by the periodate ion. Br J Exp Pathol. 1951 Feb;32(1):34–48. [PMC free article] [PubMed] [Google Scholar]
- MORGAN W. T. A contribution to human biochemical genetics; the chemical basis of blood-group specificity. Proc R Soc Lond B Biol Sci. 1960 Feb 2;151:308–347. doi: 10.1098/rspb.1960.0002. [DOI] [PubMed] [Google Scholar]
- MORRISON W. L., NEURATH H. Proteolytic enzymes of the formed elements of human blood. I. Erythrocytes. J Biol Chem. 1953 Jan;200(1):39–51. [PubMed] [Google Scholar]
- MOSKOWITZ M., CARB S. Surface alteration and the agglutinability of red cells. Nature. 1957 Nov 16;180(4594):1049–1050. doi: 10.1038/1801049a0. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M., TREFFERS H. P. An agglutinin in normal sera for periodate-treated red cells. Science. 1950 Jun 30;111(2896):717–719. doi: 10.1126/science.111.2896.717. [DOI] [PubMed] [Google Scholar]
- MUSCHEL L. H., PIPER D. R. Enzyme-treated red blood cells of sheep in the test for infectious mononucleosis. Am J Clin Pathol. 1959 Sep;32:240–244. doi: 10.1093/ajcp/32.3.240. [DOI] [PubMed] [Google Scholar]
- McCREA J. F. Studies on influenza virus receptor substance and receptor-substance analogues. II. Isolation and purification of a mucoprotein receptor substance from human erythrocyte stroma treated with pentane. Yale J Biol Med. 1953 Dec;26(3):191–210. [PMC free article] [PubMed] [Google Scholar]
- McDONALD C. E., BALLS A. K. On the esterolytic activities of papain and trypsin. J Biol Chem. 1957 Nov;229(1):69–76. [PubMed] [Google Scholar]
- Morton J. A., Pickles M. M. The Proteolytic Enzyme Test for Detecting Incomplete Antibodies. J Clin Pathol. 1951 May;4(2):189–199. doi: 10.1136/jcp.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON R. A., Jr The immune-adherence phenomenon; a hypothetical role of erythrocytes in defence against bacteria and viruses. Proc R Soc Med. 1956 Jan;49(1):55–58. [PMC free article] [PubMed] [Google Scholar]
- NETER E. Bacterial hemagglutination and hemolysis. Bacteriol Rev. 1956 Sep;20(3):166–188. doi: 10.1128/br.20.3.166-188.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEVO A., DE VRIES A., KATCHALSKY A. Interaction of basic polyamino acids with the red blood cell. I. Combination of polylysine with single cells. Biochim Biophys Acta. 1955 Aug;17(4):536–547. doi: 10.1016/0006-3002(55)90416-9. [DOI] [PubMed] [Google Scholar]
- Owen R. D. IMMUNOGENETIC CONSEQUENCES OF VASCULAR ANASTOMOSES BETWEEN BOVINE TWINS. Science. 1945 Oct 19;102(2651):400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- PETHICA B. A., SCHULMAN J. H. The physical chemistry of haemolysis by surface-active agents. Biochem J. 1953 Jan;53(2):177–185. doi: 10.1042/bj0530177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIROFSKY B., CORDOVA M. S., IMEL T. L. The nonimmunologic reaction of globulin molecules with the erythrocyte surface. Vox Sang. 1962;7:334–347. doi: 10.1111/j.1423-0410.1962.tb03262.x. [DOI] [PubMed] [Google Scholar]
- PLESCIA O. J., CAVALLO G., AMIRAIAN K., HEIDELBERGER M. Aspects of the immune hemolytic reaction. IV. Inhibition of hemolysis by the reaction products. J Immunol. 1958 May;80(5):374–381. [PubMed] [Google Scholar]
- PULSFORD M. F. Factors affecting the lysis of erythrocytes treated with staphylococcal beta toxin. Aust J Exp Biol Med Sci. 1954 Jun;32(3):347–352. doi: 10.1038/icb.1954.37. [DOI] [PubMed] [Google Scholar]
- RACE R. R., SANGER R., SELWYN J. G. A possible deletion in a human Rh chromosome; a serological and genetical study. Br J Exp Pathol. 1951 Apr;32(2):124–135. [PMC free article] [PubMed] [Google Scholar]
- REBERS P. A., HURWITZ E., HEIDELBERGER M., ESTRADA-PARRA S. Immunochemistry of the pneumococcal types II, V, and VI. III. Tests with derivatives of the specific polysaccharides of types II and VI. J Bacteriol. 1962 Feb;83:335–342. doi: 10.1128/jb.83.2.335-342.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENTON P. H., HANCOCK J. A. Uptake of A and B antigens by transfused group O erythrocytes. Vox Sang. 1962;7:33–38. doi: 10.1111/j.1423-0410.1962.tb03226.x. [DOI] [PubMed] [Google Scholar]
- RIDEAL E., TAYLOR F. H. On haemolysis by anionic detergents. Proc R Soc Lond B Biol Sci. 1956 Mar 26;146(923):225–241. doi: 10.1098/rspb.1957.0007. [DOI] [PubMed] [Google Scholar]
- ROSENFIELD R. E., VOGEL P. The identification of hemagglutinins with red cells altered with trypsin. Trans N Y Acad Sci. 1951 Apr;13(6):213–220. doi: 10.1111/j.2164-0947.1951.tb00042.x. [DOI] [PubMed] [Google Scholar]
- ROYAL G. C., FERGUSON L. C., SUTTON T. S. Bovine erythrocyte antigens. III. The isolation of blood-group specific substances from erythrocytes. J Immunol. 1953 Jul;71(1):22–25. [PubMed] [Google Scholar]
- SCHEINBERG S. L., RECKEL R. Effects of oestrogen on somatic variation of an erythrocyte agglutinogen in chickens. Nature. 1961 Jan 28;189:295–296. doi: 10.1038/189295a0. [DOI] [PubMed] [Google Scholar]
- SPAUN J. Determination of Salmonella typhi O and Vi antibodies by haemagglutination. Acta Pathol Microbiol Scand. 1952;31(4):462–469. doi: 10.1111/j.1699-0463.1952.tb00214.x. [DOI] [PubMed] [Google Scholar]
- SPRINGER G. F., RAPAPORT M. J. Specific release of heterogenetic mononucleosis receptor by influenza viruses, receptor destroying enzyme and plant proteases. Proc Soc Exp Biol Med. 1957 Oct;96(1):103–107. doi: 10.3181/00379727-96-23405. [DOI] [PubMed] [Google Scholar]
- SPRINGER G. F., WILLIAMSON P. Immunochemical significance of L- and D-fucose derivatives. Biochem J. 1962 Nov;85:282–291. doi: 10.1042/bj0850282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINGER G. F., WILLIAMSON P. Limitations of heterologous reagents in the elucidation of blood group H(O) specific structures. Vox Sang. 1963 Mar-Apr;8:177–195. doi: 10.1111/j.1423-0410.1963.tb03292.x. [DOI] [PubMed] [Google Scholar]
- STEWART W. B., PETENYI C. W., ROSE H. M. The survival time of canine erythrocytes modified by influenza virus. Blood. 1955 Mar;10(3):228–234. [PubMed] [Google Scholar]
- Springer G. F., Ansell N. J. INACTIVATION OF HUMAN ERYTHROCYTE AGGLUTINOGENS M AND N BY INFLUENZA VIRUSES AND RECEPTOR-DESTROYING ENZYME. Proc Natl Acad Sci U S A. 1958 Feb;44(2):182–189. doi: 10.1073/pnas.44.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormont C. Acquisition of the J Substance by the Bovine Erythrocyte. Proc Natl Acad Sci U S A. 1949 May;35(5):232–237. doi: 10.1073/pnas.35.5.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SØRENSEN A. N., RENDEL J., STONE W. H. The J substance of cattle. II. A comparison of normal antibodies and antigens in sheep, cattle and man. J Immunol. 1954 Dec;73(6):407–414. [PubMed] [Google Scholar]
- TOMCSIK J., BAUMANN-GRACE J. B. Action of proteolytic enzymes on the "mononucleosis antigen" in sheep and beef erythrocytes. Pathol Microbiol (Basel) 1960;23:172–183. doi: 10.1159/000160930. [DOI] [PubMed] [Google Scholar]
- TOMCSIK J., SCHERRER-GERVAI M. [The effect of neutral salts on erythrocyte membranes]. Pathol Microbiol (Basel) 1961;24:945–953. [PubMed] [Google Scholar]
- TORP H. E. Has the enzyme system acetylcholine-cholinesterase any significance for physiological hemolysis in the spleen? Scand J Clin Lab Invest. 1956;8(1):84–85. doi: 10.3109/00365515609049251. [DOI] [PubMed] [Google Scholar]
- TURNER J. C. Absence of lecithin from the stromata of the red cells of certain animals (ruminants), and its relation to venom hemolysis. J Exp Med. 1957 Mar 1;105(3):189–193. doi: 10.1084/jem.105.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UHLENBRUCK G., SCHMID D. O. [A mucoid with blood group properties from bovine erythrocyte stroma]. Z Immun exp ther. 1962 Aug;123:466–472. [PubMed] [Google Scholar]
- VAN LOGHEM J. J., Jr, DORFMEIER H., VAN DER HART M. Two A antigens with abnormal serologic properties. Vox Sang. 1957 Jan;2(1):16–24. doi: 10.1111/j.1423-0410.1957.tb03429.x. [DOI] [PubMed] [Google Scholar]
- WALLACE J. H., DODD M. C., WRIGHT C. S. Antigenic studies of virus- and trypsin-treated erythrocytes. J Immunol. 1955 Feb;74(2):89–95. [PubMed] [Google Scholar]
- WATKINS W. M., MORGAN W. T. Further observations on the inhibition of blood-group specific serological reactions by simple sugars of known structure. Vox Sang. 1962;7:129–150. doi: 10.1111/j.1423-0410.1962.tb03238.x. [DOI] [PubMed] [Google Scholar]
- WATKINS W. M., MORGAN W. T. Inactivation of the H receptors on human erythrocytes by an enzyme obtained from Trichomonas foetus. Br J Exp Pathol. 1954 Apr;35(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- WATKINS W. M., MORGAN W. T. Inhibition by simple sugars of enzymes which decompose the blood-group substances. Nature. 1955 Apr 16;175(4459):676–677. doi: 10.1038/175676a0. [DOI] [PubMed] [Google Scholar]
- WEEDEN A. R., DATTA N., MOLLISON P. L. Adsorption of bacteria on to red cells leading to positive antiglobulin reactions. Vox Sang. 1960 Dec;5:523–531. doi: 10.1111/j.1423-0410.1960.tb04038.x. [DOI] [PubMed] [Google Scholar]
- WHEELER W. E., LUHBY A. L., SCHOLL M. L. L. The action of enzymes in hemagglutinating systems. II. Agglutinating properties of trypsin-modified red cells with anti-Rh sera. J Immunol. 1950 Jul;65(1):39–46. [PubMed] [Google Scholar]
- WIENER A. S., GORDON E. B., COHEN L. A new rare Rhesus agglutinogen. Am J Hum Genet. 1952 Dec;4(4):363–372. [PMC free article] [PubMed] [Google Scholar]
- WIENER A. S., GORDON E. B. Quantitative test for antibody-globulin coating human blood cells and its practical applications. Am J Clin Pathol. 1953 May;23(5):429–446. doi: 10.1093/ajcp/23.5.429. [DOI] [PubMed] [Google Scholar]
- WIENER A. S., KATZ L. Studies on the use of enzyme-treated red cells in tests for Rh sensitization. J Immunol. 1951 Jan;66(1):51–66. [PubMed] [Google Scholar]
- WIENER A. S., RUSSOW E. Observations on the nature of the auto-antibodies in a case of acquired hemolytic anemia. Ann Intern Med. 1957 Jul;47(1):1–9. doi: 10.7326/0003-4819-47-1-1. [DOI] [PubMed] [Google Scholar]
- WOERNER H. Wirkungen des Tannins auf immunologische Vorgänge. I. Eigene Untersuchungen zur Agglutinationsbeeinflussung. Zentralbl Bakteriol Orig. 1956 Jun;166(2):100–133. [PubMed] [Google Scholar]
- WOLLNER D. Eine verbesserte Methode zur serologischen Diagnose der infektiösen Mononukleose. Klin Wochenschr. 1955 Oct 15;33(39-40):940–941. doi: 10.1007/BF01472889. [DOI] [PubMed] [Google Scholar]
- WOLPERS C. Elektronenmikroskopische Untersuchungen der Inenstrukturen kernloser Erythrocyten. II. Befunde bei einem Fall von Sichelzellanämie. Klin Wochenschr. 1957 Jan 15;35(2):57–62. doi: 10.1007/BF01490530. [DOI] [PubMed] [Google Scholar]
- WOLPERS C. Elektronenmikroskopische Untersuchungen der Innenstrukturen kernloser Erythrocyten. I. Reticulocyten und Pseudoreticulocyten. Klin Wochenschr. 1956 Jan 15;34(3-4):61–69. doi: 10.1007/BF01467161. [DOI] [PubMed] [Google Scholar]
- YAMAKAWA T., IIDA T. Immunochemical study on the red blood cells. I. Globoside, as the agglutinogen of the ABO system on erythrocytes. Jpn J Exp Med. 1953 Aug;23(4):327–331. [PubMed] [Google Scholar]
- YAMAKAWA T., YOKOYAMA S., HANDA N. Chemistry of lipids of posthemolytic residue or stroma of erythrocytes. XI. Structure of globoside, the main mucolipid of human erythrocytes. J Biochem. 1963 Jan;53:28–36. doi: 10.1093/oxfordjournals.jbchem.a127654. [DOI] [PubMed] [Google Scholar]





