Figure 4.
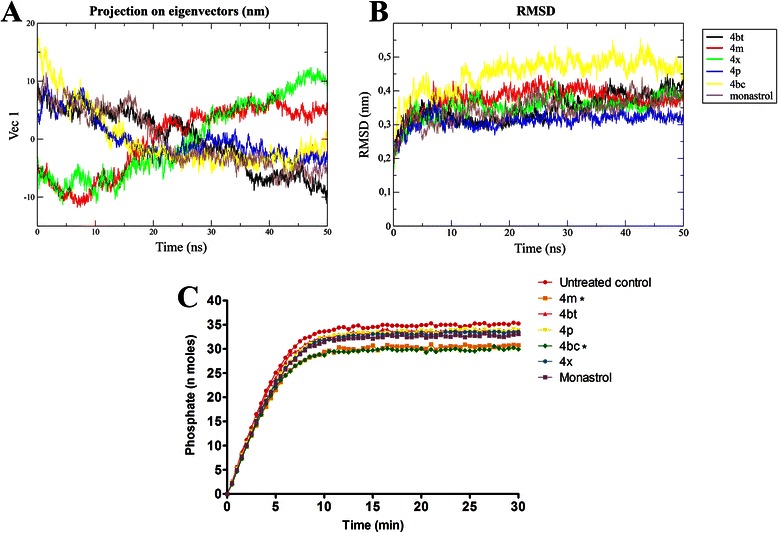
4bc leads Eg5 to a very restricted conformation and potently inhibits this protein in vitro.A, Essential dynamics analysis over time was performed to assess the DHPMs interference on Eg5 protein stability. Values far from zero mean that the structure has greater movement amplitude in its first principal component. It is possible to note that compound 4bc leads the protein to a more stable conformation after the MD assays. B, RMSD from the initial structure over time. Compound 4bc allows the greatest initial shift compared to the other molecules, but all of them lead the protein to a stable conformation after about 15 ns. C, Inhibitory rate of Eg5 by DHPMs in vitro. Kinesin Eg5 (1 μg) was incubated with the IC50 of 4 m, 4bt (dimethylenastron), 4p, 4bc, 4x and monastrol with readings taken immediately after incubation at room temperature at 30 second intervals for a total reaction time of 30 minutes. Reactions were measured in spectrophotometer set in kinetic mode and an absorbance wavelength of 360 nm. Dots, mean of nmols of phosphate versus reaction time. *P < 0.05.
