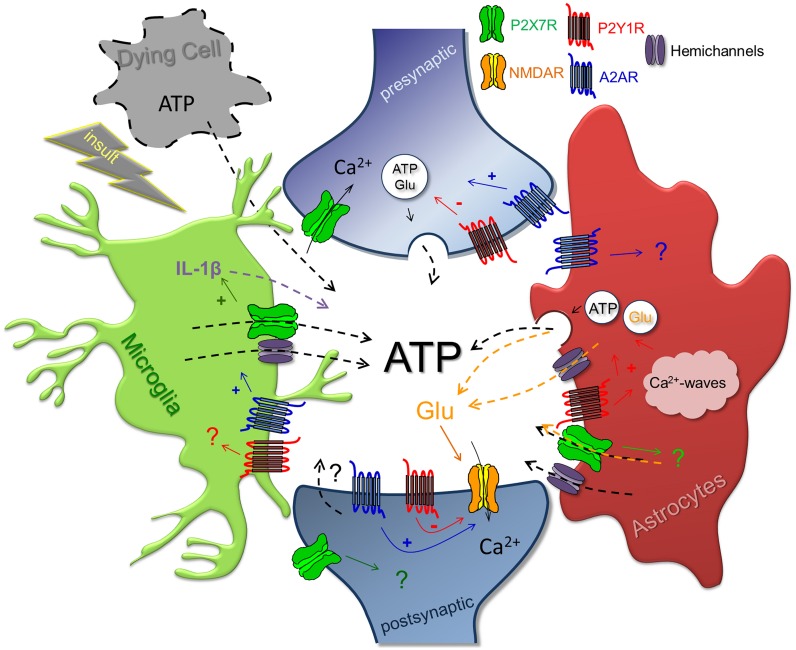
Figure 1.
Integrated view of the purinergic signaling in brain disorders. In addition to the leakage of ATP through damaged cell membrane from injured or dying cells, evolution has assured multiple mechanisms from different sources to place ATP in the extracellular milieu as a danger signal in the brain. Interestingly, this increase is self-sustained: activation of P2X7R induces the release of ATP either directly through its channel or by exocytotic or non-exocytotic mechanisms (e.g., hemichannels); P2Y1R induces the release of ATP from astrocytes; A2AR controls the release of ATP from microglia and presynaptic terminals. Once in the extracellular millieu, ATP seems to contribute to neurotoxicity through an integrated action through P2X7R, P2Y1R, and A2AR. P2X7R: it is well-established that P2X7R antagonism is beneficial by preventing the neurotoxic processing and release of IL-1β from microglia; yet a deleterious action through astrocytes namely through the regulation of glutamate levels or pro-inflammatory cytokines, or a direct neurotoxic action cannot be discarded. P2Y1R: the contribution of P2Y1R to brain demises has been mainly associated to astrocytic reactivity through Ca2+-waves and through an astrocytic-driven release of glutamate; this may be further promoted by direct actions on neuronal and synaptic function. A2AR: there is gain of function of A2AR particularly targeted to synapses in different brain disorders, where A2AR either with a presynaptic or postsynaptic locus of action, has been associated to synaptic dysfunction/loss; the precise mechanisms remain to be identified.