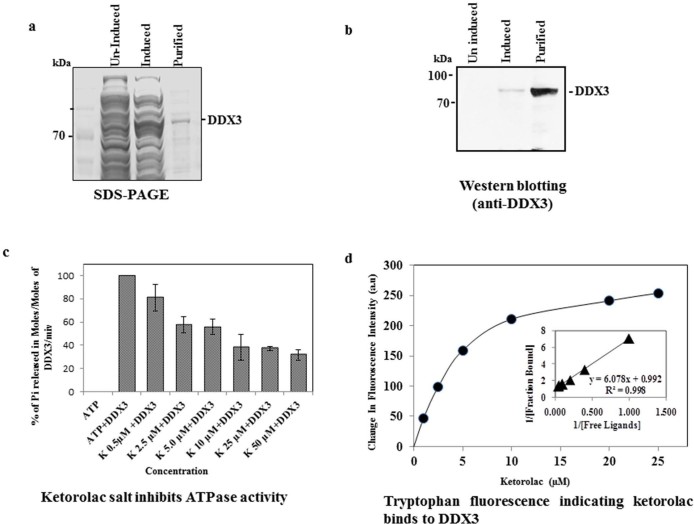
Figure 5. Binding assay for His-DDX3 to Ketorolac salt.
a) SDS-PAGE and coomassie staining showing uninduced, induced and purified His-DDX3. b) Western blot was performed using polyclonal anti-DDX3 antibody. c) Binding of Ketorolac salt to His-DDX3 results in decreased ATPase activity. 6 µM of His-DDX3 was incubated in the absence and with increasing concentration (0.5 µM to 50 µM) of Ketorolac salt. The Moles of Pi released per Moles of His-DDX3 per minute was determined by standard Malachite Green Amoniummolybdate assay as described in Materials and Methods. d) Binding of Ketorolac salt to His-DDX3. 1.5 µM of His-DDX3 was incubated without or with increasing concentration (1 µM, 2.5 µM,5 µM,10 µM,20 µM and 25 µM) of Ketorolac salt. The tryptophan emission intensity was measured at 345 nm and a gradual reduction in fluorescent intensity was observed. (Insert) The dissociation constant for the interaction between Ketorolac salt and His-DDX3 was obtained by the double reciprocal plot (kd: 6.0±0.5 µM).