Figure 4. The EPIYT site at B-TPM of CagA is phosphorylated and necessary for interaction with PI3-kinase.
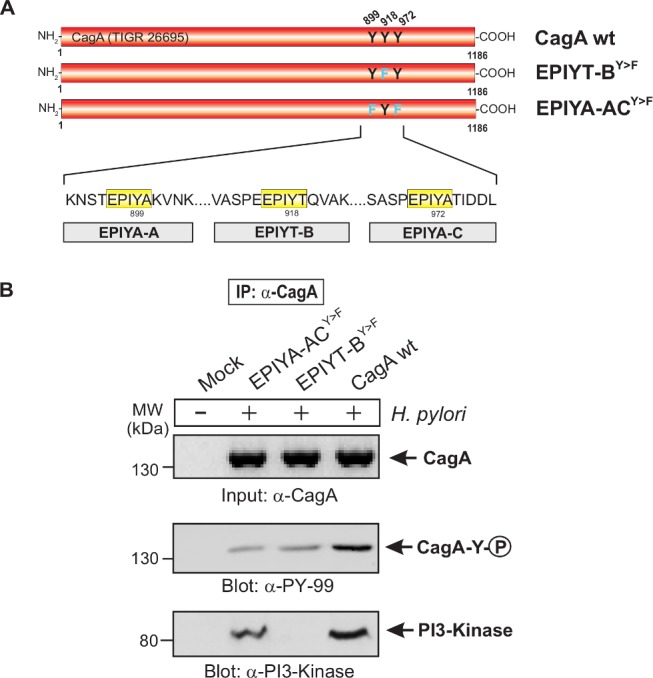
Panel A: Site-directed mutagenesis of CagA TPM-motifs A, B and C was performed to generate the indicated phospho-resistant variants. Tyrosine residues in adjacent TPM-motifs were replaced by phenylalanines. The resulting single and double mutants are indicated and complemented into the H. pylori ΔcagA mutant. Panel B: AGS cells were co-cultured with the various CagA-expressing H. pylori strains for 6 h as indicated. Cell extracts were harvested and subjected to immunoprecipitation (IP) using α-CagA antibodies. CagA phosphorylation in the IPs was examined using α-pY-99 and α-CagA antibodies (arrows). All strains expressed similar amounts of CagA, and H. pylori expressing CagA wild-type (wt), EPIYT-ACY>F, and EPIYT-BY>F all showed phosphorylation signal. Western blotting using α-PI3-kinase antibody revealed that only CagA wt and EPIYT-ACY>F can bind to PI3-kinase, but not the EPIYT-BY>F mutant, suggesting that EPIYT-B is phosphorylated and necessary for the interaction.
