Summary
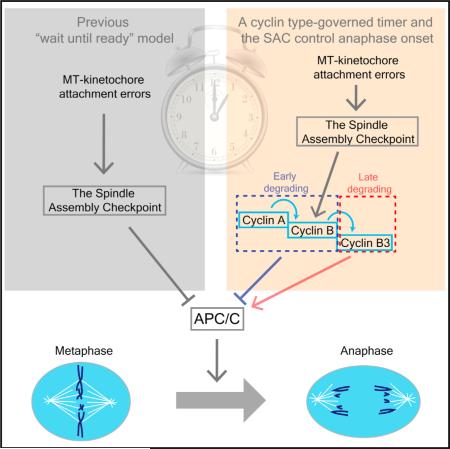
The timing mechanism for mitotic progression is still poorly understood. The spindle assembly checkpoint (SAC), whose reversal upon chromosome alignment is thought to time anaphase [1–3], is functional during the rapid mitotic cycles of the Drosophila embryo; but its genetic inactivation had no consequence on the timing of the early mitoses. Mitotic cyclins—Cyclin A, Cyclin B, and Cyclin B3—influence mitotic progression and are degraded in a stereotyped sequence [4–11]. RNAi knockdown of Cyclins A and B resulted in a Cyclin B3-only mitosis in which anaphase initiated prior to chromosome alignment. Furthermore, in such a Cyclin B3-only mitosis, colchicine-induced SAC activation failed to block Cyclin B3 destruction, chromosome decondensation, or nuclear membrane re-assembly. Injection of Cyclin B proteins restored the ability of SAC to prevent Cyclin B3 destruction. Thus, SAC function depends on particular cyclin types. Changing Cyclin B3 levels showed that it accelerated progress to anaphase, even in the absence of SAC function. The impact of Cyclin B3 on anaphase initiation appeared to decline with developmental progress. Our results show that different cyclin types affect anaphase timing differently in the early embryonic divisions. The early-destroyed cyclins—Cyclins A and B—restrain anaphase-promoting complex/cyclosome (APC/C) function, whereas the late-destroyed cyclin, Cyclin B3, stimulates function. We propose that the destruction schedule of cyclin types guides mitotic exit by affecting both Cdk1 and APC/C, whose activities change as each cyclin type is lost.
Graphical Abstract
Results and Discussion
Mitotic Cyclins, but Not the SAC, Time Metaphase-Anaphase Transition in Drosophila Early Embryos
Work in tissue culture cells suggested a “wait-until-ready” model for the control of anaphase onset [1, 3], wherein unattached chromosomes activate the spindle assembly checkpoint (SAC) to prevent anaphase-promoting complex/cyclosome (APC/C) activation until all the chromosomes are attached (i.e., ready for anaphase). A shortcoming of this mechanism appears in a multinucleate cell where the first spindle to satisfy the SAC activates anaphase in the entire cell (Figure 1A) [12]. Since this activation by the lead nucleus short-circuits regulation in all slower nuclei, this mode of timing control appears inappropriate for the syncytial Drosophila embryo.
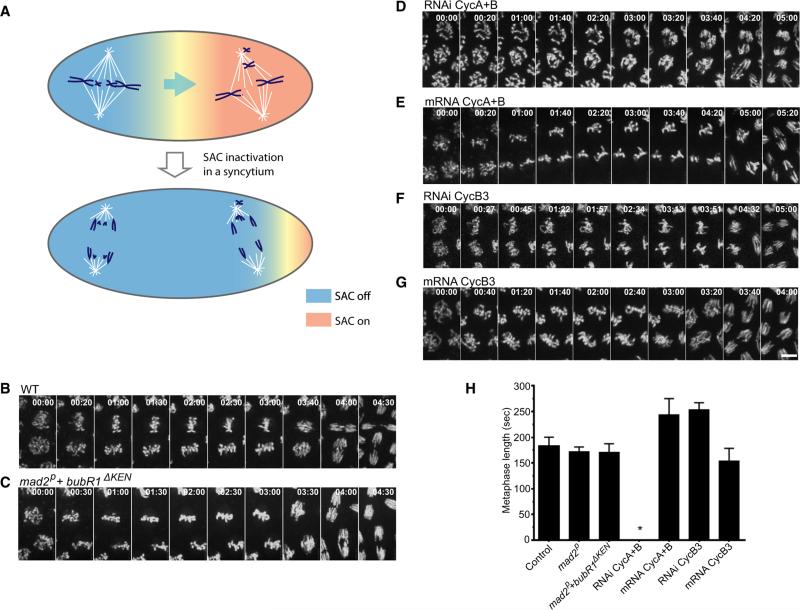
Figure 1. Mitotic Cyclins, but Not the SAC, Dictate Timing of Anaphase Onset.
(A) Schematic showing that checkpoint release by the first aligned spindle hurries the slower prometaphases into anaphase in a syncytium.
(B–G) Mitotic progress was visualized in real time by following H2AvD-GFP produced from a transgene (B, D–G), or from injected mRNA (C). Frames from videos at the indicated times (min:s) show mitosis 13 beginning at prometaphase. The time lapse to the beginning of chromosome separation reveals the timing of metaphase-anaphase in the different genotypes and injected embryos. Scale bar, 5 mμ.
(H) Comparison of metaphase duration. SAC-deficient embryos did not differ significantly from wild-type (unpaired t test, control versus mad2p: p = 0.0752; control versus mad2p+bubR1ΔKEN: p = 0.0929). Changing the levels of particular cyclins had a cyclin-type-specific effect on metaphase duration. No obvious metaphase was seen in Cyclin A- and B-depleted embryos (star), whereas a prolonged metaphase was observed when the level of Cyclin A and B was increased by mRNA injection (unpaired t test, p < 0.0001). Knockdown of Cyclin B3 extended metaphase (unpaired t test, p < 0.0001), and Cyclin B3 mRNA injection slightly shortened metaphase (unpaired t test, p = 0.0124). Error bars represent the SD.
The mitotic cyclins are degraded in an orderly sequence, with Cyclin A disappearing in metaphase, Cyclin B near the time of onset of anaphase, and Cyclin B3 during anaphase. Moreover, destruction of each cyclin is required for progress to the next stage of mitosis [4–11, 13]. Hence, the schedule of destruction ought to pace the progress of mitotic exit. To test the basis of timing control of anaphase initiation during the syncytial mitotic cycles of early Drosophila embryos, we inactivated SAC or manipulated levels of the different mitotic cyclins and analyzed the duration of metaphase (Figure 1).
The function of the SAC is dispensable for Drosophila, as mad2 null and bubR1ΔKEN mad2 double mutants were viable and fertile. Although loss of the SAC slightly accelerated progress to anaphase in larval neuroblasts [14, 15], we detected no difference in metaphase length in early embryos (Figures 1B, 1C, and 1H). Thus, the SAC did not contribute to the timing of metaphase-anaphase transition at this developmental stage.
As reported previously, RNAi knockdown of the three mitotic cyclins in embryos blocks the cell cycle rapidly and effectively [16], and knockdown of individual or pairs of cyclins gives distinct phenotypes [17, 18]. Knocking down the early-degraded cyclins, Cyclin A and Cyclin B, accelerated progress to anaphase and led to mitoses without metaphase. Embryos entered anaphase prematurely and chromosomes were randomly segregated (Figure 1D) [17]. Reciprocally, injection of mRNA encoding these early-degraded cyclins delayed chromo-some segregation (Figures 1E and 1H). To our surprise, Cyclin B3 knockdown moderately extended metaphase, and injection of CyclinB3 mRNA slightly advanced anaphase (Figures 1F–1H). We conclude that the early-degraded cyclins delay anaphase, whereas Cyclin B3 advances it.
How do the early cyclins inhibit anaphase entry? The APC/C system has a poorly understood capacity to degrade different substrates in an orderly progression [9, 19], and Cyclin A and Cyclin B are among the most preferred substrates. These cyclins enjoy several cyclin-type-specific interactions that promote their early recruitment to the APC/C [20]. These include a binding interaction between cyclin-dependent kinase regulatory subunit 1 (Cks1) and phosphorylated APC/C [20–22] and complex interactions with a category of APC/C inhibitory proteins, Rca1/Emi1/Emi2 [23]. The strong interactions may commit APC/C to these preferred substrates to enforce ordered destruction. As long as the APC/C is preoccupied with destruction of these early cyclins, its action on other substrates will be inhibited, deferring their destruction. If the early cyclins suppress APC/C-mediated destruction of later substrates, perhaps they also contribute to checkpoint suppression of APC/C activity.
SAC-Mediated Stabilization of Cyclin B3 Depends on the Presence of Early Cyclins
To investigate cyclin influence on SAC function, we treated embryos with colchicine after RNAi knockdown of pairs of cyclins (Figure 2A). Control embryos treated with colchicine stably arrested with condensed chromosomes and had no detectable nuclear membrane (Figures 2B and S2A; Movies S1 and S2). Embryos with only Cyclin B also exhibited persistent chromosome condensation (Figure S2C; Movie S1). The chromosomes in embryos with only Cyclin A started to decon-dense after a moderate arrest (Figure S2B; Movie S1), consistent with the previously reported continued degradation of Cyclin A at a checkpoint arrest [8, 24]. The chromosomes of embryos with only Cyclin B3 began to decondense after a short arrest (Figure S2D; Movie S1), and nuclear membrane staining appeared (Figure 2C; Movie S2). We conclude that spindle disruption does not stably arrest a Cyclin B3-only mitosis despite the expectation that SAC should stabilize this late-degrading cyclin.
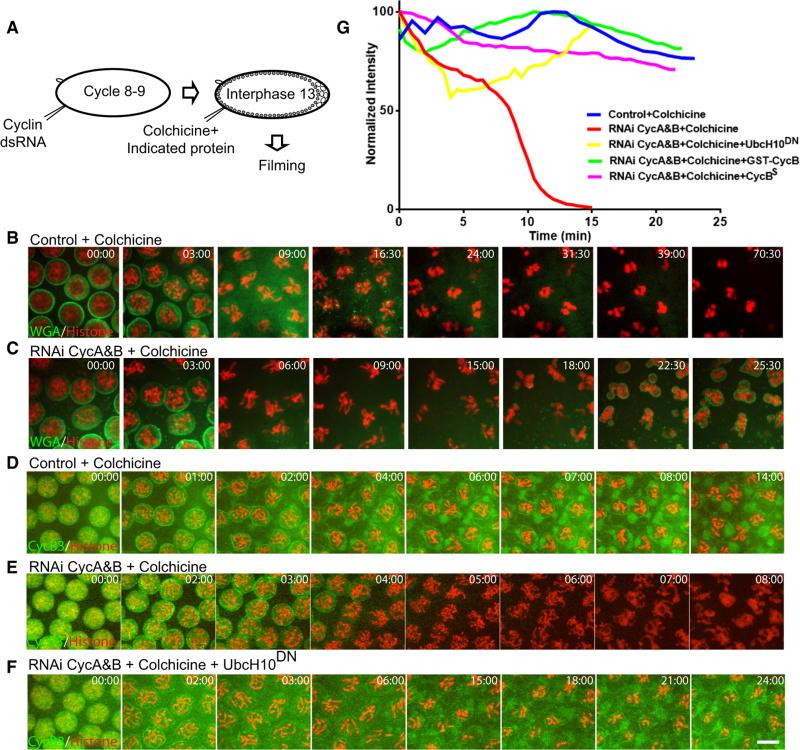
Figure 2. Full Stabilization of Cyclin B3 Requires Both the SAC and Early Cyclins.
(A) Schematic of the experiments showing the approximate stage of each injection.
(B and C) Colchicine injection induced mitotic arrest in control, but not in Cyclin A+B knockdown, embryos. Mitotic chromosomes were visualized by H2AvD-RFP (red), and nuclear envelope was visualized by injection of fluorescently labeled wheat germ agglutinin (WGA) (green). Compare based on indicated timing (min:s) not alignment.
(D–F) Characterization of Cyclin B3 degradation under different conditions. Cyclin B3-GFP (green) was stabilized by colchicine in wild-type, but not in Cyclin A+B-depleted, embryos. APC/C inhibitor UbcH10C114S blocked this destruction. Mitotic chromosomes were visualized by H2AvD-RFP (red). Note that in (E) chromosomes exited from mitosis after Cyclin B3-GFP degradation. Scale bar, 5 mμ.
(G) Quantitation of Cyclin B3-GFP fluorescent intensity after colchicine injection. Total fluorescence of each frame was measured, normalized, and plotted against time (minutes into mitosis). Cyclin B3-GFP was similarly stabilized by injection of recombinant Cyclin B proteins and a known inhibitor of the APC/C, UbcH10C114S.
See also Figures S1 and S2 and Movie S2.
The failure of SAC in the Cyclin B3-only mitosis might reflect an inability to activate the spindle checkpoint. However, Mad2 recruitment to the prometaphase kinetochores, a hallmark of SAC activation, still occurs in the Cyclin B3-only mitosis (Figure S1). This focused our attention on the function of the SAC.
Since a stable form of Cyclin B3 arrests cells in late mitosis with condensed chromosomes [9], escape from a mitotic arrest ought to be associated with Cyclin B3 destruction. To characterize cyclin degradation, we made mRNAs encoding mitotic cyclins with an EGFP tag fused to their C termini. Cyclin A-GFP and Cyclin B-GFP were enriched on centrosomes and kinetochores in mitosis (Figure S2E). Interestingly, Cyclin B3-GFP was enriched on nuclear envelope/ER-like membranous structures that bracket the spindle in early Drosophila mitoses (Figure S2F) [25]. Unlike Cyclin A, Cyclin B3 was stabilized in colchicine-injected wild-type embryos (Figure 2D). However, when Cyclin A and Cyclin B were knocked down, Cyclin B3 was degraded in the presence of colchicine (Figure 2E). Injection of recombinant Cyclin B proteins into these embryos blocked Cyclin B3 degradation (Figure 2G). Degradation of Cyclin B3 during a Cyclin B3-only mitosis was mediated by APC/C, as inhibition of APC/C activity by injecting UbcH10C114S [26, 27] prevented its destruction (Figure 2F). We conclude that SAC needs the early cyclins in order to stabilize the late-degrading Cyclin B3.
The prevailing model for SAC is not sufficient to explain why the SAC requires early-degrading cyclins. Substrate is recruited to APC/C via the interaction between the destruction box of substrate and the WD40 domains of Cdc20. The SAC, once activated, is thought to function by blocking substrate binding to Cdc20 [3, 28]. SAC inhibition of Cdc20 should inhibit Cyclin B3 destruction without reliance on an early cyclin. Apparently, the inhibition of APC/C by the SAC is more complex. We suggest that Cyclin B continues to engage the APC/C during an SAC arrest, perhaps via Cdc20-independent interactions, and that SAC suppresses the ability of APC/C to degrade Cyclin B, effectively freezing ordered degradation and holding the APC/C in abeyance.
Cyclin B3 Promotes Metaphase-Anaphase Transition in Early Drosophila Embryos
Cyclin B3 knockdown extended metaphase. Perhaps, this is the result of activation of SAC upon Cyclin B3 knockdown. We repeated the Cyclin B3 RNAi experiment in the mad2 null embryos. Cyclin B3 knockdown still caused a metaphase delay in the SAC-deficient embryos (Figure 3A).
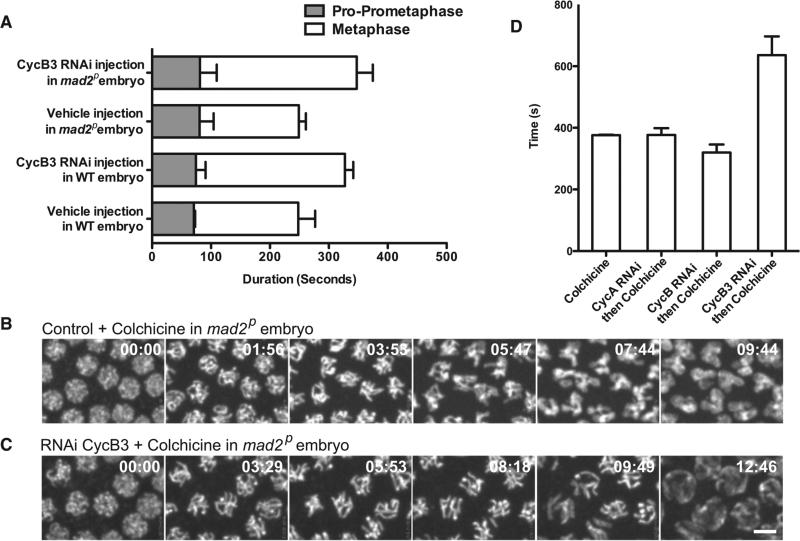
Figure 3. Cyclin B3 Promotes the Metaphase-Anaphase Transition.
(A) The metaphase extension observed in Cyclin B3 RNAi-treated embryos was SAC independent, as a similar delay in metaphase was observed in SAC-deficient mad2p embryos (unpaired t test, control versus Cyclin B3 RNAi in mad2p embryos, p < 0.0001). Error bars represent the SD.
(B and C) Colchicine treatment in mad2p embryos with or without Cyclin B3 knockdown. Chromosomes were visualized by an H2AvD-GFP transgene. Mitotic exit was delayed in Cyclin B3 RNAi-treated embryos (chromosome decondensation at 07:44 in control versus 12:46 in Cyclin B3 RNAi-treated embryos). Scale bar, 5 mμ.
(D) Duration of mitotic phase in the Cyclin B3 knockdown embryos was significantly longer than that in either control or the other cyclin RNAi-treated embryos (unpaired t test, p < 0.0001). Error bars represent the SD.
Although independent of SAC, we thought that Cyclin B3 might alter early mitotic events with secondary effects on the APC/C and transition to anaphase. The mad2 mutant offered a context in which we could disrupt the spindle and normal events of mitotic progress with colchicine and still assess mitotic exit (Figure 3B; Movie S3). To test whether Cyclin B3 influences mitotic progression in these embryos, we knocked down Cyclin B3 and followed the duration of the “mitotic phase” based on the degree of DNA condensation (Movie S3). Cyclin B3 knockdown, but not knockdown of the other cyclins, extended this mitotic phase (Figures 3C and 3D). We conclude that Cyclin B3 normally promotes the mitotic exit program and that this action is independent of both SAC and the spindle.
If Cyclin B3 directly regulates APC/C function, it might influence its cellular dynamics. Cdc27 is an APC/C subunit whose phosphorylation by Cdk1-cyclin appears to activate APC/C function and direct it to anaphase chromosomes [29]. We analyzed the anaphase localization of Cdc27 in both wild-type and single cyclin RNAi-treated embryos. Cdc27 was recruited to anaphase chromosomes in wild-type, and Cyclin A or Cyclin B knockdown embryos; however, Cyclin B3 knockdown greatly reduced the anaphase chromosomal localization of Cdc27 (Figure S3).
Cyclins generally inhibit mitotic exit. At least their destruction underlies downregulation of the mitotic Cdk, and their stabilization blocks mitotic exit [9]. This makes acceleration of anaphase onset by Cyclin B3 appear incongruous. However, cyclins are thought to promote their own demise by activating the APC/C, and the best APC/C activator among the mitotic cyclins will promote mitotic exit. Suppression of APC/C by early-degraded cyclins and activation by a late-degraded cyclin would first stabilize the metaphase state, but initial cyclin destruction would ramp up APC/C activity in a decisive transition. As a late-degrading cyclin that does not block anaphase, Cyclin B3 is particularly well suited as an activator of the APC/C.
We do not know how Cyclin B3 stimulates anaphase. However, cyclin:Cdk kinase activity phosphorylates certain APC/C subunits, such as Cdc27, as well as phosphorylating and inactivating an inhibitor of the APC/C, Emi2 [30, 31]. We suggest that Cyclin B3:Cdk1 has unique substrate specificity [32] and spatial-temporal distribution that make it an effective activator of APC/C. Regardless of the detailed mechanism, the study here shows that cyclin types influence APC/C function, and hence regulate their own destruction schedule.
Anaphase Promotion Activity of Cyclin B3 Is Developmentally Regulated
Flies without zygotic Cyclin B3 can develop into healthy adults, but the females are infertile [33]. Cyclin B3 null females had normal ovaries and laid fertilized eggs, but these exhibited early cell-cycle defects. We hypothesized that the female sterility, or more properly maternal-effect lethality, in the Cyclin B3 null flies resulted from a deficiency in exit from mitosis or meiosis.
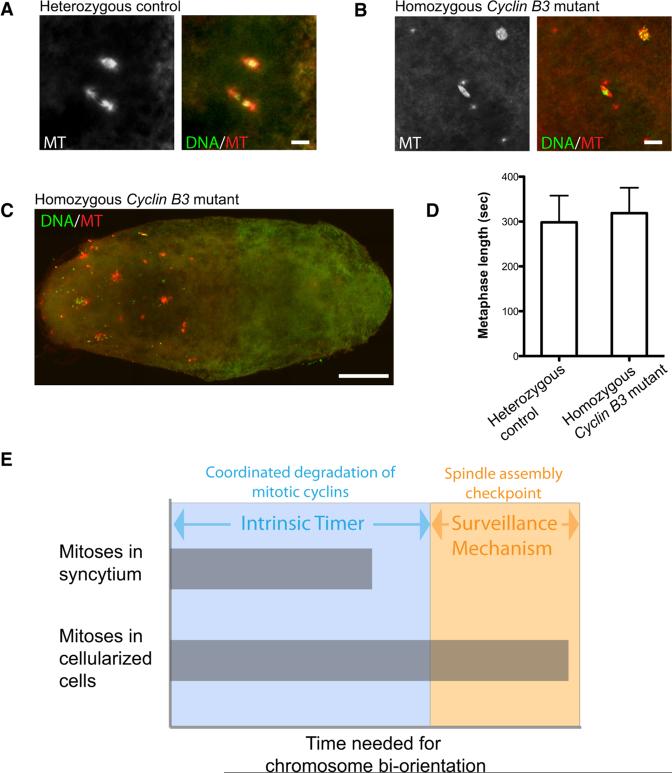
To test this, we imaged eggs from homozygous Cyclin B3 mutant mothers and controls. Eggs from Cyclin B3 null mothers were reported to be defective in exiting from meiosis [33]. However, we observed some eggs with centrosomes and polar bodies, indicators of fertilization and completion of meiosis, respectively. Figure 4B shows a Cyclin B3-deficient egg with a seemingly normal polar body and a metaphase spindle with four centrosomes adjacent to it. These centrosomes were detached from the spindle, and the metaphase spindle was acentrosomal. In the older eggs, numerous micro-tubule-organizing centers were formed and DNA became highly fragmented (Figure 4C). Our data suggested that some of the mutant eggs finished meiosis but then arrested in metaphase of the subsequent mitotic division. These observations are consistent with a role of Cyclin B3 in stimulation of anaphase as we see in later-staged embryos. We suggest that Cyclin B3 is so important in meiosis and in the earliest mitoses that its absence disrupts restoration of interphase at these stages.
Figure 4. Developmental Regulation of Cyclin B3's Activity in Promoting Anaphase Onset.
(A and B) Representative images of young embryos from heterozygous and homozygous Cyclin B3 mutant females. Scale bar, 5 mμ.
(C) An image of an older embryo from homozygous Cyclin B3 mutant females. Microtubules are shown in red and DNA in green. Scale bar, 100 mμ.
(D) No significant metaphase delay was observed in neuroblast cells from Cyclin B3 null third-instar larvae (unpaired t test, p = 0.3954). Error bars represent the SD.
(E) Metaphase-anaphase transition is timed by a cyclin-based intrinsic timer and a backup checkpoint. Since there is an intrinsic timer that paces the progress of mitotic events, a safety mechanism that stalls progress in response to improperly aligned chromosomes is of no consequence if alignment is achieved before the intrinsic timer has lapsed. However, fluctuations in the time required for alignment or of the pace of the intrinsic timer might occur as a result of environmental stress or as part of normal development. As soon as the intrinsic timer fails to accommodate chromosome alignment, the checkpoint will automatically take over regulation without requiring a significant change in regulatory arrangement.
Next, we analyzed Cyclin B3 function in larval neuroblasts, a more differentiated cell type that gives rise to neurons. Surprisingly, the metaphase duration in the neuroblasts of Cyclin B3 null larvae was indistinguishable from that in the control heterozygous siblings (Figure 4D; Movies S4 and S5). We thus conclude that the impact of Cyclin B3 on anaphase onset is developmentally regulated. Meiosis and early embryonic mitoses need Cyclin B3, but later neuroblast divisions do not. Interestingly, the constitution of the APC/C holoenzyme is also under developmental regulation. During female meiosis and early embryonic mitoses, a distinct Cdc20-like protein, CORT, is expressed [34]. It would be of great interest to explore the interaction between Cyclin B3 and this novel APC/C activator.
The lack of an effect of Cyclin B3 in later cell cycles is not due to lack of Cyclin B3. It is expressed widely in Drosophila and was shown to regulate onset of cytokinesis in later cycles [13]. This suggests that Cyclin B3 is not always important for timing and that timing duty shifts from a program of cyclin destruction at early stages to a SAC-dependent wait-until-ready mechanism. This raises the issue of how these two mechanisms are controlled to guarantee accurate mitotic progress. While it might seem dangerous to have different mechanisms timing mitosis come and go, it should be recalled that the checkpoint mechanism is beautifully appropriate as a backup mechanism. We suggest that whenever spindle establishment slows to the point that misaligned chromosomes are still present after the cyclin-based timer has run its course, the SAC takes over (Figure 4E). By removing the danger of misregulation, the SAC may have freed evolution and development to alter timing inputs governing mitotic progress.
Supplementary Material
Highlights.
Metaphase-anaphase transition in early Drosophila embryo is not timed by the SAC
Cyclins A and B inhibit anaphase entry, whereas late-degrading Cyclin B3 promotes it
SAC suppression of APC/C function depends on Cyclin B
The anaphase promotion activity of Cyclin B3 is developmentally regulated
Acknowledgments
We thank Bill Sullivan, Roger Karess, Kim Nasmyth, Jordan Raff, Todd Nystul, the Bloomington Stock Center, and Developmental Studies Hybrid-oma Bank for reagents. We gratefully acknowledge previous and current members of the P.H.O. lab for technical assistance and helpful discussions. The constructive comments from the anonymous reviewers greatly improved our manuscript and we appreciate that. This research was supported by NIH grant GM037193 to P.H.O.
Footnotes
Supplemental Information
Supplemental Information includes Supplemental Experimental Procedures, three figures, and five movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2015.01.053.
References
- 1.de Medina-Redondo M, Meraldi P. The spindle assembly checkpoint: clock or domino? Results Probl. Cell Differ. 2011;53:75–91. doi: 10.1007/978-3-642-19065-0_4. [DOI] [PubMed] [Google Scholar]
- 2.Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 4.Lehner CF, O'Farrell PH. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitfield WG, Gonzalez C, Maldonado-Codina G, Glover DM. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990;9:2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigrist S, Jacobs H, Stratmann R, Lehner CF. Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J. 1995;14:4827–4838. doi: 10.1002/j.1460-2075.1995.tb00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Elzen N, Pines J. Cyclin A is destroyed in prometa-phase and can delay chromosome alignment and anaphase. J. Cell Biol. 2001;153:121–136. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parry DH, O'Farrell PH. The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr. Biol. 2001;11:671–683. doi: 10.1016/s0960-9822(01)00204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoblich JA, Lehner CF. Synergistic action of Drosophila cyclins A and B during the G2-M transition. EMBO J. 1993;12:65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahal R, Amon A. Mitotic CDKs control the metaphaseanaphase transition and trigger spindle elongation. Genes Dev. 2008;22:1534–1548. doi: 10.1101/gad.1638308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieder CL, Khodjakov A, Paliulis LV, Fortier TM, Cole RW, Sluder G. Mitosis in vertebrate somatic cells with two spindles: implications for the metaphase/anaphase transition checkpoint and cleavage. Proc. Natl. Acad. Sci. USA. 1997;94:5107–5112. doi: 10.1073/pnas.94.10.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Echard A, O'Farrell PH. The degradation of two mitotic cyclins contributes to the timing of cytokinesis. Curr. Biol. 2003;13:373–383. doi: 10.1016/s0960-9822(03)00127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buffin E, Emre D, Karess RE. Flies without a spindle checkpoint. Nat. Cell Biol. 2007;9:565–572. doi: 10.1038/ncb1570. [DOI] [PubMed] [Google Scholar]
- 15.Rahmani Z, Gagou ME, Lefebvre C, Emre D, Karess RE. Separating the spindle, checkpoint, and timer functions of BubR1. J. Cell Biol. 2009;187:597–605. doi: 10.1083/jcb.200905026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCleland ML, O'Farrell PH. RNAi of mitotic cyclins in Drosophila uncouples the nuclear and centrosome cycle. Curr. Biol. 2008;18:245–254. doi: 10.1016/j.cub.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCleland ML, Farrell JA, O'Farrell PH. Influence of cyclin type and dose on mitotic entry and progression in the early Drosophila embryo. J. Cell Biol. 2009;184:639–646. doi: 10.1083/jcb.200810012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan K, Farrell JA, O'Farrell PH. Different cyclin types collaborate to reverse the S-phase checkpoint and permit prompt mitosis. J. Cell Biol. 2012;198:973–980. doi: 10.1083/jcb.201205007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu D, Hsiao JY, Davey NE, Van Voorhis VA, Foster SA, Tang C, Morgan DO. Multiple mechanisms determine the order of APC/C substrate degradation in mitosis. J. Cell Biol. 2014;207:23–39. doi: 10.1083/jcb.201402041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Zon W, Ogink J, ter Riet B, Medema RH, te Riele H, Wolthuis RM. The APC/C recruits cyclin B1-Cdk1-Cks in prometaphase before D box recognition to control mitotic exit. J. Cell Biol. 2010;190:587–602. doi: 10.1083/jcb.200912084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Fiore B, Pines J. How cyclin A destruction escapes the spindle assembly checkpoint. J. Cell Biol. 2010;190:501–509. doi: 10.1083/jcb.201001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolthuis R, Clay-Farrace L, van Zon W, Yekezare M, Koop L, Ogink J, Medema R, Pines J. Cdc20 and Cks direct the spindle checkpoint-independent destruction of cyclin A. Mol. Cell. 2008;30:290–302. doi: 10.1016/j.molcel.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Kirschner MW. Emi1 preferentially inhibits ubiquitin chain elongation by the anaphase-promoting complex. Nat. Cell Biol. 2013;15:797–806. doi: 10.1038/ncb2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su TT, Campbell SD, O'Farrell PH. Drosophila grapes/ CHK1 mutants are defective in cyclin proteolysis and coordination of mitotic events. Curr. Biol. 1999;9:919–922. doi: 10.1016/s0960-9822(99)80399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stafstrom JP, Staehelin LA. Dynamics of the nuclear envelope and of nuclear pore complexes during mitosis in the Drosophila embryo. Eur. J. Cell Biol. 1984;34:179–189. [PubMed] [Google Scholar]
- 26.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira RA, Hamilton RS, Pauli A, Davis I, Nasmyth K. Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei. Nat. Cell Biol. 2010;12:185–192. doi: 10.1038/ncb2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lara-Gonzalez P, Scott MI, Diez M, Sen O, Taylor SS. BubR1 blocks substrate recruitment to the APC/C in a KEN-box-dependent manner. J. Cell Sci. 2011;124:4332–4345. doi: 10.1242/jcs.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang JY, Morley G, Li D, Whitaker M. Cdk1 phosphor-ylation sites on Cdc27 are required for correct chromosomal localisation and APC/C function in syncytial Drosophila embryos. J. Cell Sci. 2007;120:1990–1997. doi: 10.1242/jcs.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Guo Y, Yamada A, Perry JA, Wang MZ, Araki M, Freel CD, Tung JJ, Tang W, Margolis SS, et al. A role for Cdc2- and PP2A-mediated regulation of Emi2 in the maintenance of CSF arrest. Curr. Biol. 2007;17:213–224. doi: 10.1016/j.cub.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isoda M, Sako K, Suzuki K, Nishino K, Nakajo N, Ohe M, Ezaki T, Kanemori Y, Inoue D, Ueno H, Sagata N. Dynamic regulation of Emi2 by Emi2-bound Cdk1/Plk1/CK1 and PP2A-B56 in meiotic arrest of Xenopus eggs. Dev. Cell. 2011;21:506–519. doi: 10.1016/j.devcel.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Kôivomägi M, Valk E, Venta R, Iofik A, Lepiku M, Morgan DO, Loog M. Dynamics of Cdk1 substrate specificity during the cell cycle. Mol. Cell. 2011;42:610–623. doi: 10.1016/j.molcel.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs HW, Knoblich JA, Lehner CF. Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev. 1998;12:3741–3751. doi: 10.1101/gad.12.23.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pesin JA, Orr-Weaver TL. Developmental role and regulation of cortex, a meiosis-specific anaphase-promoting complex/cyclosome activator. PLoS Genet. 2007;3:e202. doi: 10.1371/journal.pgen.0030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.