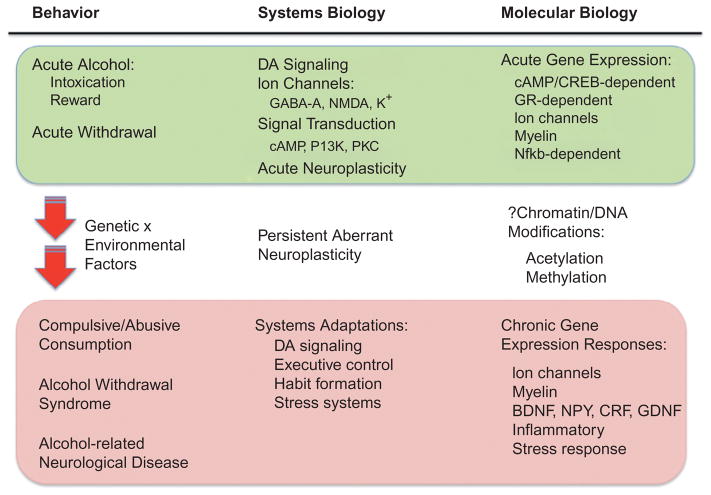
Fig. 10.1.
Overview of molecular adaptive events leading to alcoholism and neurologic complications of alcohol use disorders (AUD). The figure documents major aspects of current knowledge regarding behavioral, systems biology, and molecular mechanisms leading from acute alcohol exposure to chronic consequences such as AUD and neurologic complications. Schema are illustrative only and not meant to be all-inclusive. Acute alcohol has major direct effects on ion channels (γ-aminobutyric acid A (GABA-A), N-methyl-D-aspartate (NMDA), and K+). Alcohol-induced release of dopamine (DA) is a major factor in the rewarding properties of the drug. Alteration in numerous signaling cascades (e.g., cAMP, PI-3-kinase (PI3K), and protein kinase C (PKC)) and transcription factors (GR, glucocorticoid receptor; CREB; and Nfkb, Nf-kappa-B) may function in acute and chronic neuroplasticity by altering gene expression at the transcriptional, translational, or posttranslational level. Gene expression alterations are thought to play a central role in both acute and chronic neuroadaptation to alcohol. Epigenetic changes (DNA or histone modifications) are proposed as a mechanism underlying persistence of gene expression/neuroadaptive events, leading to clinical sequelae such as compulsive drinking, recidivism, and alcohol-related neurologic disease. BDNF, brain-derived neurotropic factor; NPY, neuropeptide Y; CRF, corticotropin-releasing factor; GDNF, glial cell-derived neurotropic factor.