Abstract
Rational
Nicotine use in schizophrenia has traditionally been explained as ‘self-medication’ of cognitive and/or nicotinic acetylcholinergic receptor (nAChR) abnormalities.
Objectives
We test this hypothesis in a neurodevelopmental rat model of schizophrenia that shows increased addiction behaviors including enhanced nicotine reinforcement and drug-seeking.
Methods
Nicotine transdermal patch (5 mg/kg/day vs. placebo × 10 days in adolescence or adulthood) effects on subsequent radial-arm maze learning (15 sessions) and frontal-cortical-striatal nAChR densities (α4β2; [3H]-epibatidine binding) were examined in neonatal ventral hippocampal lesion (NVHL) and SHAM-operated rats.
Results
NVHL cognitive deficits were not differentially affected by nicotine history compared to SHAMs. Nicotine history produced minimal cognitive effects while increasing food–reward consumption on the maze, compounding with NVHL-induced overconsumption. Acute nicotine (0.5 mg/kg) delivered before the final maze sessions produced modest improvements in maze performance in rats with nicotine patch histories only, but not differentially so in NVHLs. Consistent with in vivo neuroimaging of β2 nAChR binding in schizophrenia smokers vs. non-smokers and healthy controls, adult NVHLs showed 12% reductions in nAChR binding in MPFC (p<0.05) but not ventral striatum (<5% changes, p>.40), whereas nicotine history elevated nAChRs across both regions (>30%, p<0.001) without interacting with NVHLs. Adolescent vs. adult nicotine exposure did not alter nAChRs differentially.
Conclusions
Although replicating nicotine-induced up-regulation of nAChRs in human smokers and demonstrating NVHL validity in terms of schizophrenia-associated nAChR density patterns, these findings do not support hypotheses explaining increased nicotine use in schizophrenia as reflecting illness-specific effects of nicotine to therapeutically alter cognition or nAChR densities.
INTRODUCTION
Nicotine addiction is the leading cause of premature death in the U.S. (Mokdad et al. 2004). It typically starts during adolescence (Chen and Millar 1998) afflicting about 20% of the general population, but 60% to 90% of people with schizophrenia (Dickerson et al. 2013) where smoking-related medical illness is responsible for the majority of all deaths (Callaghan et al. 2014). Nicotine addiction is also more severe in people with schizophrenia compared to non-mentally smokers, as they consume more cigarettes per day, extract more nicotine per cigarette, and are more refractory to nicotine cessation efforts (de Leon and Diaz 2005; Williams et al. 2005).
Psychiatric research has traditionally assumed nicotine use in schizophrenia represents self-medication, assigning therapeutic value to nicotine (Dalack et al. 1998; Leonard et al. 2007). Genetic markers in schizophrenia have been associated with nicotinic acetylcholinergic receptor (nAChR) genes or function, and cognitive or electrophysiological endophenotypes of schizophrenia may be normalized by nicotine (Harris et al. 2004; Leonard et al. 2007; Potter et al. 2006; Smith et al. 2002). However, recent studies suggest that tobacco and/or nicotine have no differential cognitive performance effects in mentally ill vs. healthy subjects, (Esterlis et al. 2014; Hahn et al. 2013; Quisenaerts et al. 2014; Roh et al. 2014), and that chronic nicotine exposure may actually worsen psychiatric and cognitive trajectories (Cavazos-Rehg et al. 2014 (in press); Counotte et al. 2011; Ernst et al. 2001; McDermott et al. 2013; Mojtabai and Crum 2013; Taylor et al. 2014). Moreover, a long-standing tradition of research on smoking and schizophrenia that endorses the self-medication hypothesis as the standard view in the field, may have inadvertently promoted acceptance and positive attitudes about smoking in the minds of clinicians and patients, contributing to a treatment culture that insufficiently diagnoses and treats nicotine addiction in mentally ill people (Callaghan et al. 2014; Prochaska et al. 2013). In the middle of this debate stands the well-replicated, but still mysterious neurobiological finding that nAChRs are decreased in schizophrenia brains (Breese et al. 2000; D'Souza et al. 2012; Durany et al. 2000; Esterlis et al. 2014; Freedman et al. 1995). The significance of this is unclear, particularly since schizophrenia patients consume high doses of nicotine, which should dose-dependently increase nAChR expression (Nguyen et al. 2004). Interpretations of this phenomenon as self-medication have proposed that patients use nicotine to up-regulate low nAChRs to ameliorate cognitive deficits (Breese et al. 2000; Durany et al. 2000).
Animal models allow experimental control and prospective testing of hypotheses about causal mechanisms linking nicotine use and mental illness in ways that are not ethical in human research. Such modeling is particularly important if it is considered that introducing chronic nicotine use may be more harmful than therapeutic, and that non-therapeutic mechanisms may be responsible for increased nicotine use in schizophrenia. No studies have examined nicotine's impact on cognition and nAChRs in animal models of schizophrenia that encompass increases in nicotine reinforcement and other addiction-related behaviors. Addressing this empirical gap, we used a widely studied schizophrenia model produced by neonatal ventral hippocampal lesions (NVHL) in rats (Lipska et al. 1993). These lesions, directly impacting a small portion of total hippocampal volume, are introduced at a neurodevelopmental stage approximate to the 2nd to 3rd trimester of human development, when genetic and environmental factors are thought to initially conspire to alter hippocampal development in schizophrenia. NVHLs produce multiple behavioral, developmental, pharmacological and biological features of schizophrenia (see (Tseng et al. 2009) for comprehensive review), that result in part from corruption of normal adolescent prefrontal cortical development and cortical-hippocampal connectivity. Key behavioral features of the model include post-adolescent onset of abnormal behaviors associated with dopaminergic tone (e.g. abnormal behavioral reactivity to novelty, stressors, and stimulant drugs) that are reducible with both typical and atypical neuroleptic agents. These symptoms emerge on top of negative (e.g. social deficits, loss of grooming) and cognitive symptoms (e.g. deficits in sensory gating, decision making, contextual working memory) that are detectible before adolescence, and are neuroleptic-resistant. NVHL rats also show increased self-administration of multiple drugs characteristically abused at high rates in schizophrenia patients including cocaine, alcohol and nicotine (Berg et al. 2011; Berg et al. 2013; Chambers and Self 2002). With respect to nicotine, NVHLs amplify the effects of experimenter-delivered nicotine injections to generate short and long-term behavioral sensitization (Berg and Chambers 2008). Similarly, NVHLs self-administer nicotine at greater rates compared to healthy rats across acquisition and maintenance stages, leading to greater nicotine-seeking behavior after nicotine access is removed (Berg et al. 2013).
This study examined nicotine's effects on subsequent cognition and nAChR expression in NVHL rats, to determine if nicotine has NVHL-specific effects in these domains. We used transdermal nicotine patch-exposure (5mg/kg/day × 10 days) previously shown to produce nicotine blood levels in rats of 60-70 ng/ml (Slawecki et al. 2005). This range is comparable to the upper extremes of ranges found in human smokers and patch users of 20-60 ng/ml (Benowitz et al. 2002; Fagerstrom and Hughes 2002) which accounts for rodent–to human dose equivalencies that require somewhat higher blood levels of the drug in the smaller species with faster metabolism (Matta et al. 2007). This exposure also produces nAChR up-regulation, and bio-behavioral changes persisting from adolescence into adulthood (Nguyen et al. 2004; Slawecki et al. 2005). While two major classes of nAChRs, α7 and α4β2, are implicated in cognition and schizophrenia, we focused on α4β2 in this initial study because it is involved in diverse behavioral effects of nicotine, including cognitive and primary reinforcing effects (Picciotto et al. 1998) and is a key receptor target of interest for treating both nicotine addiction (e.g. with varenicline) and schizophrenia (Roh et al. 2014). Assessment of nAChRs across cortical-striatal regions implicated in schizophrenia and addiction (Chambers et al. 2013) employed [3H]-epibatidine radio-ligand binding commonly used to study α4β2 receptors in nicotine use (Nguyen et al. 2004) and schizophrenia (Breese et al. 2000). In cortical-striatal regions, α4β2 receptors represent >90% of epibatidine binding (Perry et al. 2002). For measuring cognition, we employed a Radial-Arm Maze (RAM) paradigm that is sensitive to NVHL-cognitive deficits (Chambers et al. 1996) and nicotine (Levin and Rose 1991). Our study design provided an animal model parallel to recent human neuroimaging work looking at nAChRs in human smokers with and without schizophrenia (Esterlis et al. 2014) while addressing these questions: 1) Are NVHL-cognitive deficits differentially altered after chronic nicotine and/or with acute doses compared to controls? 2) Do NVHL rats show differential baseline or nicotine-induced nAChR up-regulation 3) Do nicotine-induced nAChR changes reflect age, NVHL, or nicotine-related differences in cognition?
MATERIALS AND METHODS
Subjects, Surgery and Experimental Design
Pregnant Sprague-Dawley rats (14 days gestation) arriving from Harlan (Indianapolis) were housed under standard conditions. Litters were culled to males only on Post-natal Day (PD) 4. On PD 7, pups (16-18g) were randomized to neonatal lesion vs. SHAM surgery to keep litters approximately balanced by lesion status, but with an overabundance of lesion over SHAM surgeries (ratio of lesion:sham rats approximately 1.2 per litter) to anticipate attrition due to poorly placed lesions. Ibotenate was infused into ventral hippocampi bilaterally in NVHLs while SHAMs only received vehicle, as described in elsewhere (Chambers and Lipska 2011). Post-operatively, pups were returned to their litters and left undisturbed until weaning (PD 21). Thereafter, subjects were pair-housed (by lesion) until patch exposure, then individually housed. As per the overall experimental design (Figure 1A), NVHL and SHAM rats were randomly assigned into balanced cohorts to undergo nicotine (NIC) vs. placebo (PBO) patch exposure for 10 days in adolescence (PD 35-44) or adulthood (PD 60-69). One day afterward, rats entered either the cognitive arm of the study (i.e. 15 sessions of RAM testing with acute nicotine delivered to all rats before sessions 13-15), or the binding arm (i.e. sacrifice for nAChR binding in cortical-striatal tissue samples). Procedures complied with Guide for the Care and Use of Laboratory Animals and Indiana University IACUC.
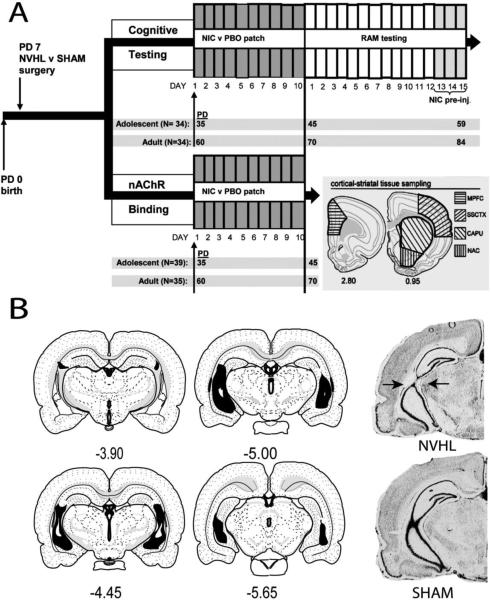
Figure 1. Experimental Design and Histology.
(A) Experimental arms and time lines according to post natal days (PD) of adolescent vs. adult NIC vs. PBO patch exposure are shown with final experiment subgroups numbers (N). Tussue for nAChR binding was dissected according to cortical-striatal regions shown at lower right. (B) Left panels depict smallest (white) and largest extents (black) of hippocampal changes in NVHL rats (n=71) included in the study. Photomicrographs (right) show NVHL vs. SHAM brains illustrating optimal NVHL damage at the confluence of ventral and dorsal blades of the hippocampus (arrows). Section maps and coordinates relative to Bregma based on Swanson(Swanson 2004).
Nicotine
Transdermal nicotine patches (Nicoderm CQ, 7 mg, Smith-Kline Beecham: Pittsburgh, PA) vs. placebo (PBO) patches (medical adhesive) were applied based on previous methods (Slawecki et al. 2005). Patches (7.17 cm2) cut based on rat weights delivered 5 mg/kg/day. The dorsum of the head was shaved for placement with re-applications occurring once daily for 10 days in adolescence (PD 35-44) or 10 days in adulthood (PD 60-69). For nicotine delivered during RAM testing, nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in 0.9% sterile saline to a stock solution of 0.5 mg/ml (expressed as base of the salt), adjusted to pH 7.4.
Cognitive Testing
Contextual-spatial learning and working memory were assessed and recorded in real time (blind to lesion status and nicotine history) using an 8-arm RAM. The RAM (Med Associates, St. Albans, VT) stood 6.5 cm above the floor with an octagonal arena (29.5 cm diameter) and eight runways extending radially (61cm × 9cm × 17 cm walls), surrounded by visual landmarks. At the beginning of all sessions, a food reward (½ Kellogg's Froot Loop) was placed at the end of all 8 arms, behind a wall 7 cm from the end of each runway, requiring rats to traverse each arm entirely to discover and consume the food reward. After initiating food-restriction (limited so that rats would never drop below 85% of their pre-test weights) one day before testing, rats were tested over 15 sessions, one session per day. In each session, rats navigated the maze from a start in the central arena to acquire Froot Loops lasting until all arms were entered (all four paws entered) or 300 seconds had expired, whichever came first. As rats learn the maze over multiple sessions, they complete the maze more efficiently both in terms of making fewer entries into arms already entered for that session, and requiring less time to complete. Rats also tend to consume more Froot loops over learning, although in normal rats, maze performance (e.g. ETR) can often exceed the number of Froot loops consumed, as some Froot loops may be found but left uneaten. Parameters of contextual-spatial working memory performance and attention to the task (efficiency) were quantified as Entries to Repeat (ETR; number of arms entered before an arm entry was repeated), and total Session Time. The number of Froot Loops eaten was also recorded as a dependent measure that provides an indication of natural reward consumption, (while understanding that rats could not eat more rewards than the number of arms they enter, and that normal rats do not always eat all the rewards they encounter. This measure is also useful for verifying that poor maze performance that could occur is not merely a result of lack of normal motivation to find and consume the food. To see if acute nicotine delivery could differentially impact cognitive performance, based on lesion status and prior chronic nicotine history, all subjects received a behaviorally active dose of nicotine (0.5 mg/kg s.c.) (Berg and Chambers 2008; Berg et al. 2013) 30 minutes before the final 3 sessions.
Receptor Binding and Lesion Verification
Rats were sacrificed under isoflourane and decapitated. Three 2 mm coronal sections, starting at the frontal pole, were cut with a Brinkman chopper from harvested brains. The medial prefrontal cortex (MPFC) was micro-dissected from the second section (Swanson 2004) while the nucleus accumbens (NAC), caudate-putamen (CAPU), and somatosensory cortex (ssCTX) were micro-dissected from the third (Figure 1A). Samples were bilaterally pooled and stored (−80 °C).
The binding protocol using [3H]-epibatidine (2 nM) began with sample homogenization with Tissue Tearor (Model 985-370, Biospec Products, Inc) in 50 mM Tris-HCl buffer, pH 7.4, at room temperature. The homogenate was centrifuged twice with a Beckman Model J2-21 Centrifuge at 4 °C and 35,000 × g for 10 minutes in fresh buffer. Pellets were re-suspended in fresh buffer and added to tubes containing Tris buffer and [3H]-epibatidine (2 nM) with or without 300 μM nicotine to a final incubation volume of 1 ml. Samples incubated for 2 hours at room temperature. Binding was terminated by filtration through GF/C glass fiber filters presoaked in 0.5% polyethylenimine to reduce nonspecific binding. Samples were then washed three times with ice-cold distilled water. The filters were placed in vials and counted with a scintillation counter. Nonspecific binding was determined in the presence of nicotine. Specific binding was calculated by subtracting nonspecific from total binding. All samples were run in duplicate with results averaged.
Brains were evaluated for appropriate lesions as described previously (Chambers and Lipska 2011), blind from behavioral results and prior to binding. Caudal brains containing the hippocampus were cryostat-cut into 40 μm coronal sections every 400 μm from approximately 3.3 to −5.8 mm (bregma), then fixed and stained with 0.5% thionin. Damage was assessed microscopically as tissue atrophy, paucity of nuclei, or cellular disarray in the ventral hippocampus. Brains with unilateral damage, damage extending into the dorsal hippocampus, or into medial or lateral structures were excluded from analysis. Exemplary histologies of rats included in the study are shown in Figure 1B (Swanson 2004). For RAM-testing, 34 SHAMs and 40 lesions were initially prepared, with 34 (85%) having acceptable lesions. For binding, 37 SHAMs and 46 lesions were initially prepared with 37 of lesions (80%) qualifying. Thus at total of N =142 rats were analyzed in the study with n=68 rats entering the cognitive arm and n=74 entering binding. In the receptor binding analysis that compared rat and analogous human data, only adult rats (n=35) were examined.
Statistics
For RAM testing, dependent measures (ETR, Session time, Froot Loops) were analyzed across the first 4 time blocks (with each block being an average of 3 consecutive once-daily sessions) to assess initial learning. Subsequently, these measures were examined across blocks 4-5 to assess acute effects of nicotine pre-injections. Four-way Repeated Measures ANOVAs examined lesion, age, and drug history over blocks. Post hoc analyses using smaller dimensional ANOVAs investigated key interactions. For binding, 3-way ANOVAs (lesion, age, drug history) were applied to receptor densities in regions separately. Additional 2-way ANOVAs of brain regions replicating analyses conducted in recent neuroimaging of α4β2 receptors in human were also performed (Esterlis et al. 2014). For this analysis, we examined adult rats only, and 2 brain regions (ventral striatum, MPFC), which corresponded to subject ages and brain regions examined in the human study (Esterlis et al also examined thalamus, parietal cortex and hippocampus, which we did not). Data are presented as means + SEM throughout text and figures, with significance at p<0.05.
RESULTS
Cognition
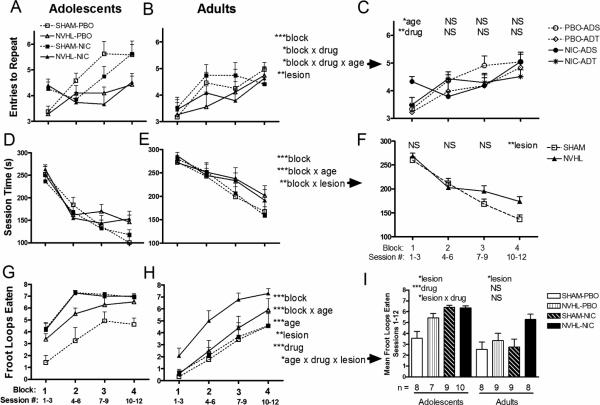
Across the first 4 blocks of RAM experience, rats demonstrated learning with increasing ETR (block: F(3,180)=18.48, p<0.001) (Figure 2A, 2B), although NVHL showed impairments (lesion: F(1, 60)=9.19, p<0.01) with a nearly significant interaction between lesion and block (F(3,180)=2.5, p=0.06). Although nicotine history had no stand-alone main effect to change ETR, it did alter the shape of the learning curve (block × drug: F(3,180)=2.77, p<0.05), flattening it somewhat, in an age-dependent way (block × age × drug: F(3, 180)=2.846, p<0.05) (Figure 2A, 2B). Post hoc testing (2-way ANOVAs: age × drug) at each block revealed this as significant effects of age (F(1,64)=4.47, p<0.05) and drug (F(1,64)=7.86, p<0.01) (Figure 2C) in the first block, with relatively better performance (increased ETR) immediately after nicotine exposure (in the first block) particularly in adolescents. Rats also increased maze performance efficiency as session times decreased overall (blocks: F(3, 180)=109.66, p<0.001) (Figure 2D, 2E), with adolescent and SHAM rats showing steeper learning curves compared to their adult (block × age: F(3, 180)=7.41, p<0.001) and NVHL counterparts (block × lesion: F(3, 180)=5.21, p<0.01). The latter interaction is more clearly visible in post-hoc ANOVAs of lesion effects at each block (Figure 2F), showing that NVHL rats were increasingly less efficient compared to SHAMS as learning progressed over the sessions reaching significance in the 4th block (lesion: (F(1,66)= 7.59, p<0.01)). Time per arm entered, as a more sensitive measure of non-specific locomotor speed on the maze (compared to session time), also decreased across the 4 blocks (F(3,180)=15.87, p<0.001). However, unlike session time, time per arm did not vary by lesion, age or nicotine history, indicating that the effects on session time were not due merely to non-specific changes in locomotor speed.
Figure 2. Cognitive testing.
ETR (A, B) Session time (D, E) and Froot loops Eaten (G, H) were analyzed separately using mixed ANOVAs (lesion, drug patch history, age, blocks) with age sorted into columns for visual clarity only. Statistical effects are presented at right of the (B) column (*p<0.05, **p<0.01, ***p<0.001, NS=non-significant), with post-hoc graphs and ANOVAs and exploring specific interactions (indicated by rightward arrows) shown in (C) column. In (C), age and drug history effects were examined at each block; in (F), lesion at each block; in (I), mean Froot Loops consumed over all blocks, were examined as effects of lesion and drug history for each age group. Subgroup numbers (n) shown below (I) are accurate to all panels.
Food-Reward Consumption
RAM learning over the first 4 blocks produced overall increases in Froot Loop consumption (blocks: F(3, 180)=110.06, p<0.001) (Figure 2G, 2H). Adolescents consumed greater amounts both in the earlier sessions (block × age: F(3, 180)=9.38, p<0.001) and overall (age: F(1, 60)=26.63, p<0.001); 5.52 ±.27/session compared to 3.45± .36 for adults. NVHL rats also showed greater consumption (F(1, 60)=11.65, p<0.01), consuming 5.12 ±.29 Froot Loops/session compared to 3.86±.39 for SHAMs, showing that their cognitive performance deficits (in terms of ETR and Session time) could not be accounted for as reflecting lack of reward motivation. As with NVHL effects, nicotine history also increased consumption (F(1, 60)=15.48, p<0.001), so that nicotine-exposed rats consumed 5.23±.33 per session compared to 3.65±.35 for PBO rats. A three way interaction of main effects was detected (F(1, 60)=5.74, p<0.05) prompting post hoc 2-way ANOVAs at each age looking at lesion and drug effects on average Froot Loops consumed (Figure 2I). NVHLs increased consumption in both adolescents (F(1,30)=5.94, p<0.05 ) and adults F(1,30)=6.49, p<0.05) while in adolescents only, nicotine history increased consumption significantly (F(1,30)=25.47, p<0.001), and more so in SHAMs than NVHLs (lesion × drug: F(1,30)=6.59, p<0.015)), potentially due to a ceiling effect limiting nicotine history increases in NVHLS .
Acute Nicotine Effects on Maze Performance
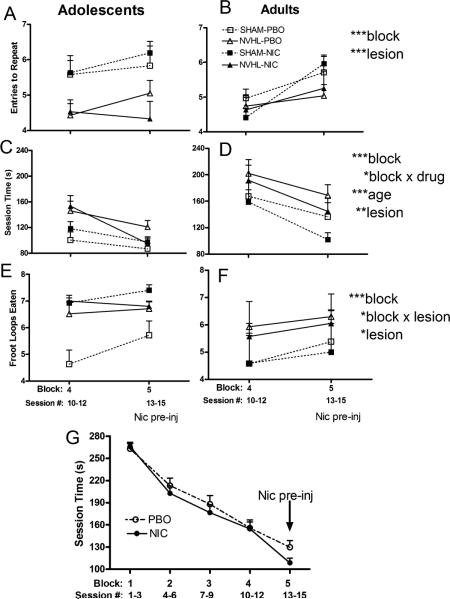
In observing whether acute nicotine injections delivered 30 minutes before each of block 5 sessions could further improve cognition, RAM performance was examined as a repeated measure from block 4 to 5 (Figure 3). ETR improved overall (block: F(1,60) =12.7, p<0.001) while NVHLs remained impaired (lesion: F(1,60) =11.7, p <0.001) without any effects or interactions of nicotine history, age, or block (lesion × drug: F(1,60)=0.1, p=0.74, lesion × block: F(1,60)=2.0, p=0.16) (Figures 3A, 3B). Session time also shortened (block: F(1,60)=53.9, p<0.001), but in a way that did depend on nicotine history (block × drug: F(1,60)=4.2, p<0.05) (Figures 3C, 3D). Post-hocs examining nicotine history at each block suggested this interaction was carried by an effect of acute nicotine to improve performance for rats with nicotine history only (contrast block 4, drug: F(1,66)=0.01, p=0.9 with block 5, drug: F(1,66)=3.7, p=0.059). Accordingly, rats with and without nicotine histories had almost identical session times in block 4 (154.4 ±9.4 vs. 155.7 ± 10.9) that separated substantially in block 5 (108.5±6.5 vs.129.7 ± 9.1). Notably, the performance effect of acute nicotine on session time cannot readily be attributed to a locomotor speed (e.g. locomotor sensitization) effect of acute nicotine injections in rats with nicotine patch histories, because, unlike total session time, average time per arm did not vary significantly as an interaction between nicotine patch history and block (F(1,60)=1.13, p=0.30). The effect of acute nicotine injections to specifically enhance performance in nicotine patch exposed animals compared to non-nicotine exposed, beyond what practice on the maze is producing, is best visualized in Figure 3G which shows session times over all 5 blocks of RAM testing. Again, although NVHL deficits in session time were significant (lesion: F(1,60)=9.96, p<0.01), acute nicotine effects did not interact with NVHLs to improve session time (lesion × drug: F(1,60)= 0.2, p=0.64 and lesion × block: F(1,60)=1.1, p=.30). Acute nicotine also did not interact with adolescent age-effects to lower session times (age:(F(1,60)=19, p<0.001)). Froot loops consumed also increased across blocks 4 to 5 (F(1,60)=14.6, p<0.001) (Figure 3E, 3F), with more consumption by NVHLs (lesion: F(1,60)=6.9, p<0.05), but in a way that varied by block (lesion × block: F(1,60)=5.2, p<0.05) suggestive of NVHL consumption nearing a ceiling effect.
Figure 3. Cognitive performance with acute nicotine pre-injections.
Block 4 to 5 performance on ETR (A, B), Session time (C, D) and Froot Loops Eaten (E, F) analyzed as mixed ANOVAs (lesion, drug patch history, age, blocks) is shown with ages sorted into columns for visual clarity. Significant main effects or interactions are presented on the right (*p<0.05, **p<0.01, ***p<0.001). In (G), data for all rats (n=68) is compiled according to NIC vs. PBO patch history groupings only, depicting the nicotine history specificity of acute nicotine pre-injections to improve Session time in NIC patch history rats only, as per the block × drug interaction in (D).
Receptor Binding
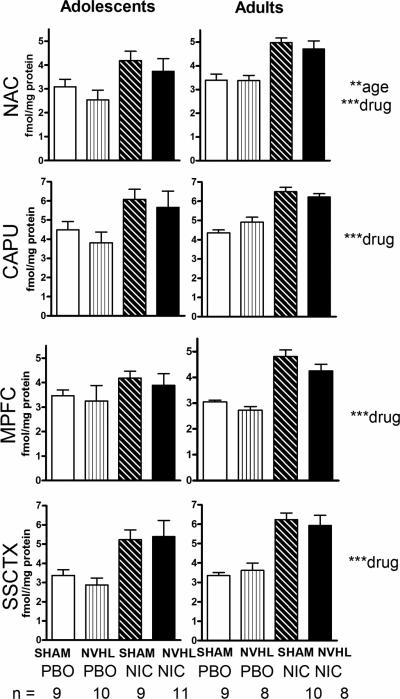
Baseline and nicotine-induced nAChR expression across brain regions are shown Figure 4. In the NAC, adolescents showed lower nAChR expression (age: F(1,66)=8.0, p<0.01). Rats with nicotine exposure had elevated nAChR densities in the NAC (F(1,66)=25.3, p<0.001) (42% increase), CAPU (F(1,66)=23.3, p<0.001) (42% increase), MPFC (F(1,66)=20.0, p<0.001) (36% increase) and SSCTX (F(1,66)= 46.1, p<0.001) (73% increase).
Figure 4. nAChR binding densities.
The 4 cortical striatal regions were analyzed in separate 3-way ANOVAs (lesion, drug history, age) with ages parsed into left and right columns for visual clarity. Significant effects are presented at right (**p<0.01, ***p<0.001). Subgroup numbers (n) are accurate to all panels in the figure.
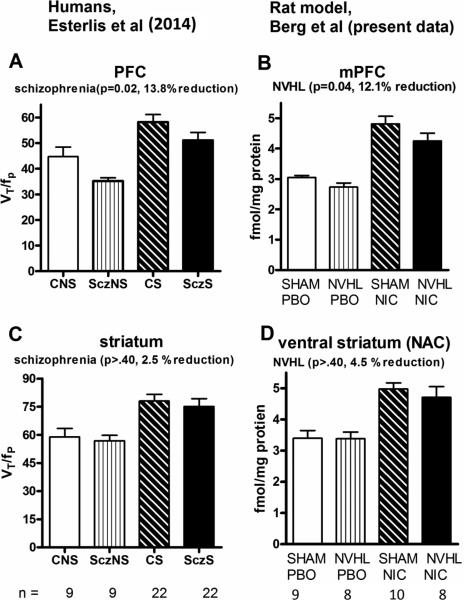
Although in 13 out of 16 comparisons (same age/drug history) NVHLs had lower nAChR densities vs. SHAMs, lesion effects in the initial 3-way analysis were not significant. There also were no significant interactions between nicotine effects and lesion in any region (drug × lesion/NAC: F(1,66)=0.02, p=0.89; CAPU: F(1,66)=0.15, p=0.70; MPFC: F(1,66)=0.10, p=0.75; SSCTX: F(1,66)= 0.002, p=0.96). However in secondary analyses focusing on MPFC and striatal regions in adult rats only, consistent with regions and statistical approaches used in recent nAChR imaging in humans (Esterlis et al. 2014) (Figure 5A, 5C), NVHLs reduced α4β2 binding (lesion:(F(1,31)=4.7, p<0.05) in the MPFC by 12.1% (Figure 5B), paralleling 13.8 % decreases in human schizophrenia, without producing significant modulation in the ventral striatum ((lesion/NAC: F(1,31)=0.33, p=0.57) (Figure 5D) or dorsal striatum (lesion/CAPU: F(1,31)=0.44, p=0.51), where lesion based changes in nAChR densities were <5% different from SHAMs. Also consistent with the human data (Esterlis et al. 2014), this analysis showed nicotine-induced increases in receptor densities across these regions (drug/MPFC: F(1,31)=65, p<0.001; drug/NAC: F(1,31)=33, p<0.001), but these nicotine effects did not interact with NVHLs (lesion × drug/MPFC: F(1,33)=0.38, p=0.54; lesion × drug/NAC: F(1,33)=0.24, p=0.63).
Figure 5. nAChR binding densities in adult rats in NAC and MPFC.
A comparison of α4β2 densities in human (in vivo imaging) (Esterlis et al. 2014) (A, C) vs (present rat data) (D, E) focuses on comparable regions, ages and analytic approach (2-way ANOVA (schizophrenia vs. nicotine histories). Control Non-smokers (CNS) compare with SHAM-PBO; Schizophrenia Non-smokers (SczNS) with NVHL-PBO; Control Smokers (CS) with SHAM-NIC; and Schizophrenia Smokers (SczS) with NVHL- NIC. Subgroup n's for the respective human and animal studies are depicted at bottom.
DISCUSSION
NVHLs show schizophrenia-like deficits in contextual-working memory with prior nicotine patch exposure having little impact on subsequent cognition, and no specific effects on the cognition of NVHLs vs. controls. Similarly, although acute nicotine injections did have some effect to enhance efficiency of maze performance, this effect was specific only to rats with prior nicotine history and was not different between NVHLs and SHAMs. Prior chronic nicotine history was nevertheless clearly pharmacologically active both behaviorally and biologically in SHAM and NVHL rats alike: Nicotine history was just as potent as the NVHL in increasing food consumption used to motivate maze learning. Nicotine also significantly increased nAChR expression, but again without interacting with NVHL effects on receptor densities. NVHLs produced down-regulation of nAChR densities that reached significance in the MPFC, but not in striatal regions. This pattern of results is in tight agreement with recent in-vivo neuroimaging of β2-nAChR receptors in schizophrenia subjects (Esterlis et al. 2014), which parallel to our own study is the first human study published thus far that uses a 2 × 2 design to examine nAChRs in mentally ill vs. healthy brains with and without nicotine history. Prior work has shown that nicotine-behavioral sensitization, and acquisition, maintenance and relapse phases of nicotine self-administration are all amplified in the NVHL model (Berg and Chambers 2008; Berg et al. 2013). Together with the present findings where biologically and behaviorally active histories of chronic nicotine exposure failed to alter NVHL- cognitive deficits, or abnormal α4β2 receptors patterns in a way that is different from healthy rats, these data do not support the self-medication explanation for increased nicotine use in schizophrenia as mediated by illness-specific therapeutic changes in cognition or nAChRs.
Multiple studies have now examined the effects of nicotine in NVHL-deficient cognition measured on the RAM (Berg et al. 2013; Chambers et al. 1996). Despite differences in dosing, routes of entry, and timing of cognitive testing relative to nicotine delivery, whether before (Chambers et al. 1996), during (Berg et al. 2013), or after (present study), the same result was observed: Schizophrenia-like cognitive deficits are present in the NVHL model, but are not uniquely nor substantially ameliorated by nicotine in a way that is different from what nicotine does in healthy rats. The present study adds weight to this conclusion by showing that while new nicotine dosing (Figure 3) impacts maze performance (session time) in subjects with prior nicotine histories, this effect was not NVHL specific. These findings agree with recent well-controlled human experiments (that account for baseline differences in smoking history) in which schizophrenia smokers vs. controls (non-smoking schizophrenia, non-mentally ill smokers, non-mentally ill non-smokers) also do not show differential cognitive responses to nicotine challenge delivered via intranasal, transdermal patches, or smoking (Hahn et al. 2013; Quisenaerts et al. 2014). Of additional translational significance, the present study showed that nicotine history and NVHLs increased consumption of the simple carbohydrate reward that baited the maze (Froot Loops). Increased appetite and weight gain are well-documented complications of smoking cessation in humans (Stamford et al. 1986), and pharmacological modulation of α4β2 receptors alter appetitive responses (Cocores and Gold 2008). Likewise, schizophrenia patients have heightened obesity risk (McCreadie 2003). Notably, the lack of substantial or specific cognitive benefits of nicotine in NVHL rats cannot be interpreted as insensitivity of NVHL rats to the pharmacological-behavioral effects of nicotine, because strong appetitive effects after cessation of nicotine history were observed in both NVHL and SHAM rats alike. Similarly, we saw no differential effects of acute nicotine injections to alter cognition in NVHL vs. SHAMs (either in terms of ETR or Session time), even though these injections do produce a significantly stronger behavioral locomotor sensitization effect in NVHLs compared to SHAMs (Berg and Chambers 2008). Still, more studies are needed to examine the cognitive effects of nicotine in alternative schizophrenia models and using cognitive paradigms alternative to the RAM to determine the generalizability of these findings. Effects of acute nicotine to temporarily improve cognition, uniquely in rats previously exposed to nicotine, should also be confirmed with experiments using vehicle injections. In terms of examining the differential effects of nicotine history on cognition due to adolescent vs. adult exposure (and their interaction with NVHLs), future studies should advance beyond the present study to vary both the developmental timing of nicotine exposure and the timing of cognitive testing.
This is the first study of nAChRs in the NVHL model or any other widely validated animal model of schizophrenia although abnormal acetylcholinergic neurotransmission has been observed in the PFC of NVHL rats after manipulation of dopamine and glutamate transmitter systems (Alexander et al. 2009; Laplante et al. 2004a; Laplante et al. 2004b). Our results show that while α4β2 receptor densities are not modulated by NVHLs as strongly as with nicotine, these receptors are down regulated by NVHLs in the MPFC, but not in underlying-striatal regions, closely paralleling human schizophrenia data (Esterlis et al. 2014). Since NVHL effects on nAChR receptor down regulation in the MPFC occurred as a consequence of early developmental hippocampal lesions in an outbred strain (e.g. without pre-selection for a specific genetic background), our results raise the intriguing possibility that nAChR expression deficits in schizophrenia may not necessarily depend directly or specifically on abnormalities in nAChR genes. Rather, such changes could be secondary to cortical-hippocampal disconnection associated with a wide variety of other genetic and environmental factors. Accordingly, low brain nAChR densities also occur in neuropsychiatric disorders other than schizophrenia, e.g. in patients with non-specific cognitive impairments and dementia (Sabri et al. 2008). A wide range of molecular, cellular, and architectural abnormalities have been identified in the MPFC of NVHL rats, including down regulations in the expression levels of multiple genes(Chambers et al. 2013; Tseng et al. 2008). Accordingly, the NVHL-based nAChR reductions we are reporting represent just one piece of an extremely complex set of biological changes in the MPFC secondary to the early developmental hippocampal insult.
The capacity of chronic nicotine exposure to up-regulate α4β2 nAChR densities we observed in the present investigation is a well replicated observation in animals and humans (D'Souza et al. 2012; Nguyen et al. 2004) indicating that the chronic dosing of nicotine we used was biologically active and appropriate to the research question being pursued. Accordingly, we found that nicotine history drove up MPFC and NAC nAChRs by 57% and 43% in MPFC and NAC regions respectively over baselines found in PBO patch rats. These are comparable to 37% and 32% increases in nAChR binding in the PFC and striatum of human smokers vs. non-smokers found by Esterlis et al (Esterlis et al. 2014). While demonstrating that the NVHL model does emulate human schizophrenia nAChR receptor patterns at least in terms of α4β2 and other receptor complexes that epibatidine binds, it remains unknown whether certain medications, abused drugs, comorbid disease states, or aging could further down-regulate nAChRs in NVHL rats or schizophrenia. Further studies using the NVHL model with alternative ligands for α4β2 and α7 receptor complexes are needed to investigate these possibilities.
Comparing nicotine effects in adolescents vs. adults tested whether cognitive or receptor modulation could depend on adolescent neurodevelopmental changes implicated in schizophrenia, addiction, or the NVHL model (Chambers et al. 2003; Weinberger 1987). The effects of nicotine history on learning did differ somewhat by age in terms of a three-way interaction between block, age, and nicotine on ETR, suggesting nicotine withdrawal has different effects in adolescents (O'Dell et al. 2004). However, this effect was subtle as it was not observed as a 2-way interaction of age and nicotine in either ETR or session time. Meanwhile, nAChRs were also not differentially modulated in any brain region by nicotine according to age of exposure. The strongest effects of age on RAM performance emerged as adolescent-increases in food consumption, and as adolescent-decreases in overall session time in the later RAM sessions. Meanwhile, only one brain region, the NAC, displayed an age-related difference of nAChR densities (regardless of nicotine exposure). Altogether, these findings do not suggest that nicotine's effects on cognition or nAChRs, in SHAM or NVHLs, are particularly dependent on adolescent vs. adult nicotine exposure. However they do hint at the possibility of a stronger interplay between adolescent neurodevelopment, nAChRs in the NAC, and nicotine's effects on natural appetitive motivation.
In summary, in line with emerging human cognitive and imaging data (Esterlis et al. 2014; Hahn et al. 2013; Quisenaerts et al. 2014; Roh et al. 2014) these results do not support hypotheses espousing nicotine as a cognitive enhancer that is specifically efficacious for schizophrenia compared to healthy brains. Further, these data do not support the idea that nicotine-induced up-regulation of nAChR densities occurs differentially in the schizophrenic brain as a basis for cognitive remediation, or as a mechanism that increases rates of nicotine use, even as MPFC densities of nAChRs are abnormally low in the disorder. NVHL rats emulate a wide range of biological and behavioral abnormalities involving prefrontal cortical-striatal circuits and altered responsiveness to several types of addictive drugs, that are characteristic of both schizophrenia and addicted populations (Chambers et al. 2013; Tseng et al. 2009). Here, we determined that although encompassing increased nicotine addiction vulnerability behaviors consistent with human schizophrenia, the NVHL model does not show differential responsiveness to the cognitive or nicotinic receptor effects of nicotine. These findings are in disagreement with widely held assumptions that beneficial-medicinal effects of nicotine are key to driving up high use rates of this drug in mental illness. However, these and related findings in the NVHL model do agree with recent human pharmacological and neuroimaging data suggesting that cortical-striatal circuit disturbances in schizophrenia generate increased nicotine addiction vulnerability (Moran et al. 2013), while the cognitive-benefits of nicotine may be subtle, transient, and not different than in healthy brains (Esterlis et al. 2014; Hahn et al. 2013; Quisenaerts et al. 2014; Roh et al. 2014). More animal and human studies that focus on mechanisms of addiction pathogenesis that are shared among addictive drugs, and accelerated in the context of mental illness, are needed for developing more effective prevention and treatment strategies for dual diagnosis disorders including schizophrenia.
ACKNOWLEDGEMENTS
The authors sincerely thank Irina Esterlis et al. for their permission to represent there comparable human data in graphical form for this manuscript (Esterlis et al. 2014). Of note, the Yale (human) and IU (animal modeling) investigative teams were not aware of each other's parallel study designs or results until after publication of Esterlis et al, and manuscript preparation of this report.
FUNDING AND DISCLOSURE
This study was supported by NSF GK-12 Doctoral Training Program Grant (SAB) and NIAAA (R01 AA020396) (EAE).
Footnotes
The authors have no conflicts of interest to report.
REFERENCES
- Alexander KS, Brooks JM, Sarter M, Bruno JP. Disruption of mesolimbic regulation of prefrontal cholinergic transmission in an animal model of schizophrenia and normalization by chronic clozapine treatment. Neuropsychopharmacology. 2009;34:2710–20. doi: 10.1038/npp.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hansson A, Jacob P., 3rd Cardiovascular effects of nasal and transdermal nicotine and cigarette smoking. Hypertension. 2002;39:1107–12. doi: 10.1161/01.hyp.0000018825.76673.ea. [DOI] [PubMed] [Google Scholar]
- Berg SA, Chambers RA. Accentuated behavioral sensitization to nicotine in the neonatal ventral hippocampal lesion model of schizophrenia. Neuropharmacology. 2008;54:1201–7. doi: 10.1016/j.neuropharm.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg SA, Czachowski CL, Chambers RA. Alcohol seeking and consumption in the NVHL neurodevelopmental rat model of schizophrenia. Behav Brain Res. 2011;218:346–9. doi: 10.1016/j.bbr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addict Biol. 2013 doi: 10.1111/adb.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–64. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Veldhuizen S, Jeysingh T, Orlan C, Graham C, Kakouris G, Remington G, Gatley J. Patterns of tobacco-related mortality among individuals diagnosed with schizophrenia, bipolar disorder, or depression. J Psychiatr Res. 2014;48:102–10. doi: 10.1016/j.jpsychires.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Cavazos-Rehg PA, Breslau N, Hatsukami D, Krauss MJ, Spitznagel EL, Grucza RA, Salyer P, Hartz SM, Bierut LJ. Smoking cessation is associated with lower rates of mood/anxiety and alcohol use disorders. Psychological Medicine. 2014;44:2523–2535. doi: 10.1017/S0033291713003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Lipska BK. A Method to the Madness: Producing the neonatal ventral hippocampal lesion rat model of schizophrenia. In: O'Donnell P, editor. Animal Models of Schizophrenia and Related Disorders. Humana Press; Totowa, N.J.: 2011. [Google Scholar]
- Chambers RA, McClintick JN, Sentir AM, Berg SA, Runyan M, Choi KH, Edenberg HJ. Cortical-striatal gene expression in neonatal hippocampal lesion (NVHL)-amplified cocaine sensitization. Genes Brain Behav. 2013;12:564–75. doi: 10.1111/gbb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–94. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–52. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. (Eng); 39-48(Fre) [PubMed] [Google Scholar]
- Cocores JA, Gold MS. Varenicline, appetite, and weight reduction. J Neuropsychiatry Clin Neurosci. 2008;20:497–8. doi: 10.1176/jnp.2008.20.4.497. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci. 2011;14:417–9. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, Pittman B, Ranganathan M, Cosgrove K, Staley J. Lower Beta-2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry. 2012;169:326–34. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155:1490–501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76:135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Schroeder J, Yolken RH. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64:44–50. doi: 10.1176/appi.ps.201200143. [DOI] [PubMed] [Google Scholar]
- Durany N, Zochling R, Boissl KW, Paulus W, Ransmayr G, Tatschner T, Danielczyk W, Jellinger K, Deckert J, Riederer P. Human post-mortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson's syndrome. Neurosci Lett. 2000;287:109–12. doi: 10.1016/s0304-3940(00)01144-7. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–9. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Ranganathan M, Bois F, Pittman B, Picciotto MR, Shearer L, Anticevic A, Carlson J, Niciu MJ, Cosgrove KP, D'Souza DC. In Vivo Evidence for beta Nicotinic Acetylcholine Receptor Subunit Upregulation in Smokers as Compared with Nonsmokers with Schizophrenia. Biol Psychiatry. 2014;76:495–502. doi: 10.1016/j.biopsych.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO, Hughes JR. Nicotine concentrations with concurrent use of cigarettes and nicotine replacement: a review. Nicotine Tob Res. 2002;4(Suppl 2):S73–9. doi: 10.1080/1462220021000032753. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry. 2013;74:436–43. doi: 10.1016/j.biopsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–85. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Laplante F, Srivastava LK, Quirion R. Alterations in dopaminergic modulation of prefrontal cortical acetylcholine release in post-pubertal rats with neonatal ventral hippocampal lesions. J Neurochem. 2004a;89:314–23. doi: 10.1111/j.1471-4159.2004.02351.x. [DOI] [PubMed] [Google Scholar]
- Laplante F, Stevenson CW, Gratton A, Srivastava LK, Quirion R. Effects of neonatal ventral hippocampal lesion in rats on stress-induced acetylcholine release in the prefrontal cortex. J Neurochem. 2004b;91:1473–82. doi: 10.1111/j.1471-4159.2004.02831.x. [DOI] [PubMed] [Google Scholar]
- Leonard S, Mexal S, Freedman R. Smoking, Genetics and Schizophrenia: Evidence for Self Medication. J Dual Diagn. 2007;3:43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rose JE. Nicotinic and muscarinic interactions and choice accuracy in the radial-arm maze. Brain Res Bull. 1991;27:125–8. doi: 10.1016/0361-9230(91)90293-s. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McCreadie RG. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534–9. doi: 10.1192/bjp.183.6.534. [DOI] [PubMed] [Google Scholar]
- McDermott MS, Marteau TM, Hollands GJ, Hankins M, Aveyard P. Change in anxiety following successful and unsuccessful attempts at smoking cessation: cohort study. Br J Psychiatry. 2013;202:62–7. doi: 10.1192/bjp.bp.112.114389. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Crum RM. Cigarette smoking and onset of mood and anxiety disorders. Am J Public Health. 2013;103:1656–65. doi: 10.2105/AJPH.2012.300911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama. 2004;291:1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moran LV, Sampath H, Kochunov P, Hong LE. Brain circuits that link schizophrenia to high risk of cigarette smoking. Schizophr Bull. 2013;39:1373–81. doi: 10.1093/schbul/sbs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. J Neurochem. 2004;90:40–9. doi: 10.1111/j.1471-4159.2004.02482.x. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:167–74. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Davila-Garcia MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J Neurochem. 2002;82:468–81. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–7. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Potter D, Summerfelt A, Gold J, Buchanan RW. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr Bull. 2006;32:692–700. doi: 10.1093/schbul/sbj050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Fromont SC, Wa C, Matlow R, Ramo DE, Hall SM. Tobacco use and its treatment among young people in mental health settings: a qualitative analysis. Nicotine Tob Res. 2013;15:1427–35. doi: 10.1093/ntr/nts343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quisenaerts C, Morrens M, Hulstijn W, de Bruijn E, Timmers M, Streffer J, De la Asuncion J, Dumont G, Sabbe B. The nicotinergic receptor as a target for cognitive enhancement in schizophrenia: Barking up the wrong tree? Psychopharmacology (Berl) 2014;231:543–50. doi: 10.1007/s00213-013-3264-9. [DOI] [PubMed] [Google Scholar]
- Roh S, Hoeppner SS, Schoenfeld D, Fullerton CA, Stoeckel LE, Evins AE. Acute effects of mecamylamine and varenicline on cognitive performance in non-smokers with and without schizophrenia. Psychopharmacology (Berl) 2014;231:765–75. doi: 10.1007/s00213-013-3286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri O, Kendziorra K, Wolf H, Gertz HJ, Brust P. Acetylcholine receptors in dementia and mild cognitive impairment. Eur J Nucl Med Mol Imaging 35 Suppl. 2008;1:S30–45. doi: 10.1007/s00259-007-0701-1. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell AK, El Khoury A, Mathe AA, Ehlers CL. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: relation to anxiety-like and depressive-like behavior. Neuropeptides. 2005;39:369–77. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–97. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Stamford BA, Matter S, Fell RD, Papanek P. Effects of smoking cessation on weight gain, metabolic rate, caloric consumption, and blood lipids. Am J Clin Nutr. 1986;43:486–94. doi: 10.1093/ajcn/43.4.486. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. 3rd edn. Elsevier; Elsevier: 2004. [Google Scholar]
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ. 2014;348:g1151. doi: 10.1136/bmj.g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O'Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–9. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis DM, Abanyie F, Steinberg ML, Foulds J, Benowitz NL. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophr Res. 2005;79:323–35. doi: 10.1016/j.schres.2005.04.016. [DOI] [PubMed] [Google Scholar]