Figure 24.
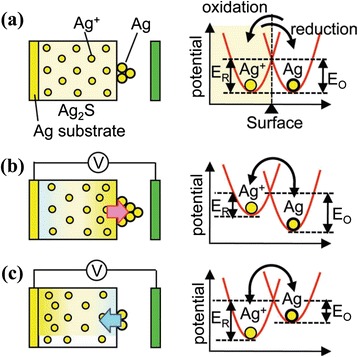
Simple schematic diagrams of the mechanism of Ag nanowire growth and dissolution process. (a) When no bias is applied, then the activation energies for reduction and oxidation are equal in the equilibrium condition. (b) Positive bias on the Ag2S electrode causes the diffusion of Ag+ cations towards the surface of the Ag2S electrode, making E R smaller than E O. As a result, the reduction rate of Ag+ cations enhances. (c) Due to the negative bias on the Ag2S electrode, Ag+ cations diffuse towards the bottom of the Ag2S electrode, making E O smaller than E R. As a result, Ag atoms oxidize easily and diffuse inside the Ag2S electrode [38].
