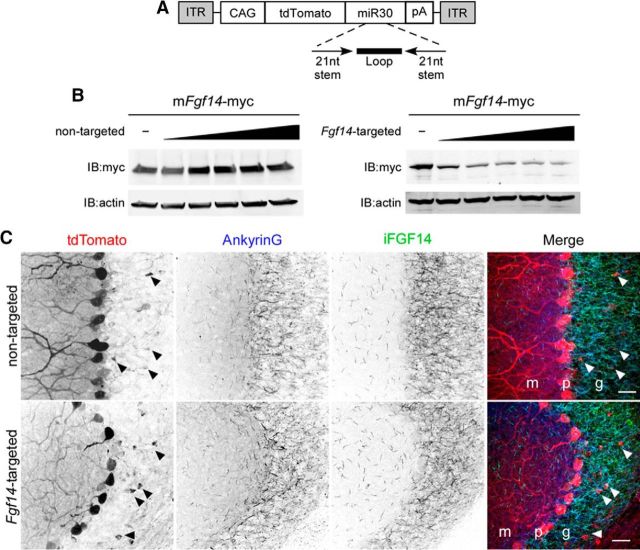
Figure 1.
Generation and validation of Fgf14-targeted shRNA. A, Schematic representation of the AAV transfer vector with an shRNA embedded in a miRNA (miR30) context in the 3′-UTR of tdTomato. Expression is driven by the chicken β-actin (with a CMV enhancer) promoter (CAG). ITR, Inverted terminal repeat; pA, polyadenylation signal. B, Mouse Fgf14-myc was expressed in CHL cells with increasing amounts of either the nontargeted or the Fgf14-targeted shRNA plasmid. Western blot analyses using an anti-myc antibody revealed that the Fgf14-targeted, but not the nontargeted, shRNA reduced iFGF14 protein expression in a dose-dependent manner. Blots were also probed with an anti-actin antibody to verify equal sample loading. C, Wild-type mice were injected into the cerebellum with the nontargeted shRNA-targeted (top) or the Fgf14-targeted (bottom) shRNA-expressing AAV1. Parasaggital sections were cut and stained with anti-Ankyrin G (blue) and anti-FGF14 (green) antibodies. In A, Representative low-magnification images of nontargeted shRNA- and Fgf14-targeted shRNA-transduced cells are shown. Viral-transduced neurons were identified by tdTomato expression and cell type was determined by morphology and location. Purkinje neurons, for example, were readily identified by large somata and extensively branched dendritic trees extending into the molecular layer; TdTomato expression in Purkinje neurons is robust. Arrowheads indicate the few tdTomato expressing (virally transduced) granule neurons. Scale bars, 25 μm. m, Molecular layer; p, Purkinje layer; g, granule layer. Overall anti-FGF14 labeling in the granule layer is similar in sections prepared after injections of the nontargeted and Fgf14-targeted shRNA-expressing AAV1.