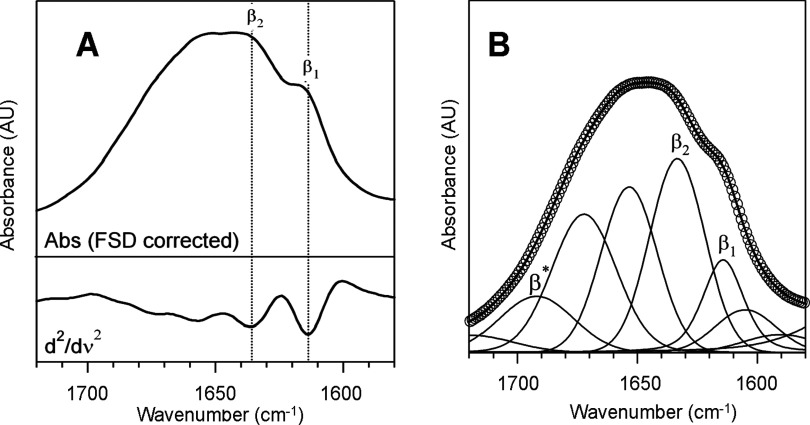
Figure 2. Secondary structure of fibrillar Hfq CTR from FTIR absorption spectroscopy.
(A) Amide I FSD corrected spectrum (top) and second-derivative spectrum (down) of C-terminal Hfq fibrils. Two β-sheet moieties, β1 and β2, are clearly detected. (B) Experimental (circles) and fitted (lines) data of amide I band of C-terminal Hfq fibrils. Two distinct β-sheet structures are present in the aggregates, β1 and β2 and the presence of the high frequency component β* suggests the possible presence of antiparallel moieties [51].