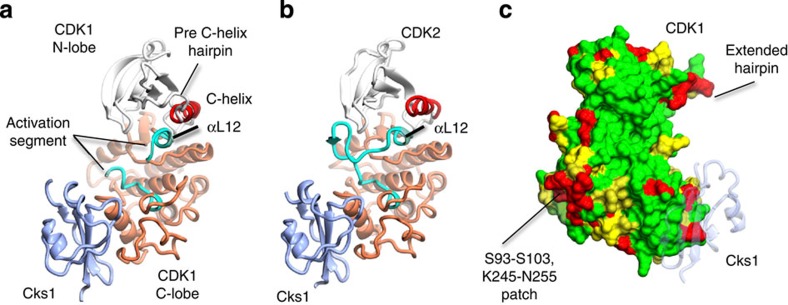
Figure 1. Structure of monomeric CDK1 bound to Cks1 and comparison with CDK2–Cks1.
The complexes are shown in an identical view in panels a and b, and rotated by circa 60 degrees around a vertical axis in panel c. (a) CDK1–Cks1, (b) CDK2–Cks1 (PDB code 1BUH,31). In both panels the CDK N- and C-terminal lobes are coloured white and coral, respectively, and the Cks subunit is coloured ice-blue. Key regulatory elements are also highlighted: C-helix (CDK1 and CDK2 residues 47–57, red) and activation segment (CDK1 and CDK2 residues 146–173 and 145–172, respectively, cyan). (c) Sequence conservation between human CDK1 and CDK2 mapped onto the surface of CDK1 bound to Cks1. Identical residues (green), conserved residues (yellow) and non-conserved (red) as defined by CCP4MG70. The structure of Cks1 is shown in semi-transparent ribbon representation to indicate the location of its binding site.