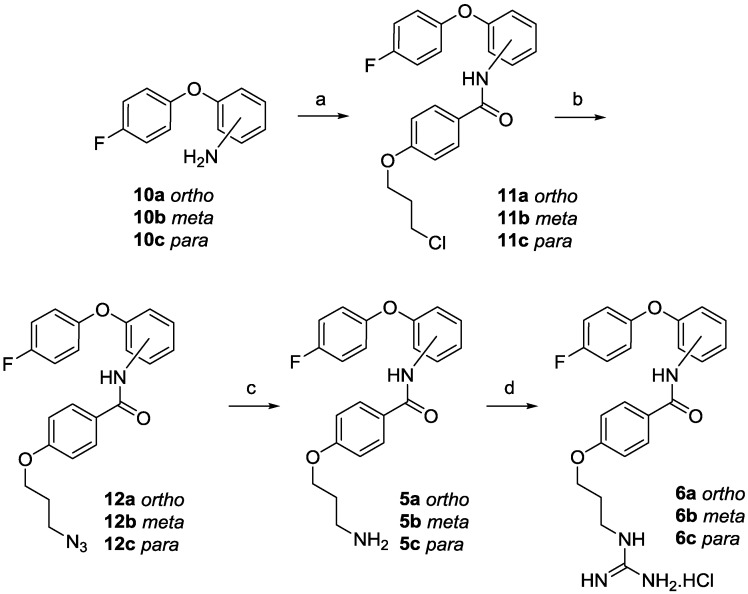
Scheme 2.
Synthesis of phenoxy anilides (5a–c and 6a–c). Reagents and conditions: (a) 4-(3-chloropropoxy)benzoic acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC·HCl), 4-dimethylaminopyridine (DMAP), Et3N, DCM/tetrahydrofuran (THF), (11a) 47%, (11b) 81% or 4-(3-chloropropoxy)benzoyl chloride, THF, (11c) 70%; (b) NaN3, dimethyl sulfoxide (DMSO), 70 °C, (12a) 95%, (12b) 99%, (12c) 92%; (c) Pd/C, NH2NH2·H2O, MeOH, (5a–c) quant.; (d) 1H-pyrazole-1-carboximidine hydrochloride, N,N-diisopropylethylamine (DIPEA), dimethylformamide (DMF), (6a–c) quant.