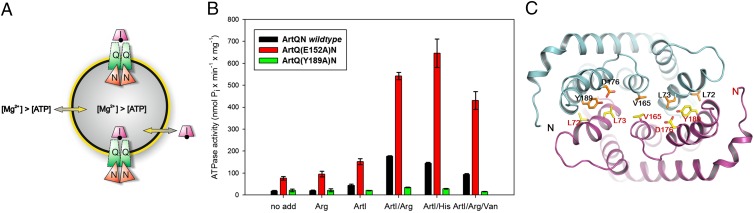
Fig. 1.
Liganded ArtI stimulates the ATPase activity of Art(QN)2. (A) Cartoon illustrating the experimental setup of the ATPase assay. Art(QN)2 is incorporated into proteoliposomes in two possible orientations: with the nucleotide-binding subunits, ArtN2, facing the medium or being exposed to the lumen of the proteoliposomes. ATPase activity is initiated by adding ArtI, Arg/His (black dot), ATP, and Mg2+ ions (in excess over ATP). Under these conditions, Mg2+ will permeabilize the proteoliposomes (24), thus allowing access of ATP and ArtI/Arg/His to the lumen. (B) Purified variants were incorporated into liposomes formed from G. stearothermophilus lipids and assayed for ATPase activity in the presence or absence of ArtI/Arg or ArtI/His. Reactions were started by adding 3 mM MgCl2 and 2 mM ATP to preheated (70 °C) proteoliposomes in the presence of 50 mM Mops (pH 7.5), ArtI (35 µM), l-arginine or l-histidine (100 µM each), and ortho-vanadate (1 mM) (where indicated). Aliquots (25 μL containing 3 μg of protein) were taken in 2-min intervals and placed into wells of a microtiter plate containing 25 μL of a 12% (wt/vol) SDS solution. The amount of liberated phosphate was determined colorimetrically with ammonium molybdate complexes using Na2HPO4 as the standard. Further details are provided in SI Methods. SE mean (n ≥ 3). (C) Ribbon diagram of TM helices surrounding with substrate-binding sites viewed along the membrane bilayer from the periplasmic side. ArtQ subunits are colored in cyan and magenta. The residues involved in dimer formation of ArtQ are shown in ball-and-stick models.