Fig. 4.
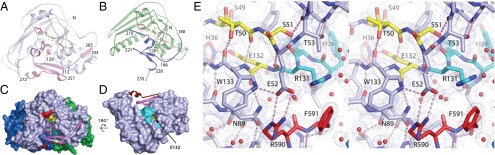
Steric blocks of CARDS TX and PTx mART active sites. The R–STS/T–E signature motif residues are colored as in Fig. 3A. (A) CARDS TX mART domain. C230 and C247 within the steric block form a disulfide bond (yellow spheres). The view is as in Fig. 2B. (B) PTx mART domain. C41 and C201 form a disulfide bond (yellow spheres) linking the steric block to the N-terminal portion of the mART domain. (C) Surface representation highlighting the steric block of the CARDS TX NAD+-binding pocket. (D) Catalytic and substrate recognition residues are buried at the D1/D2D3 interface. D2 and D3 have been removed for clarity. A tunnel into the active site runs adjacent to the ARTT motif. The yellow surface inside the tunnel is E132. (E) 1.9-Å electron density with coefficients 2mFo − DFc contoured at 1.2 σ superimposed on the refined CARDS TX structure. The C-terminal residues of the toxin, R590 and F591, project into the active site.
