Figure 1.
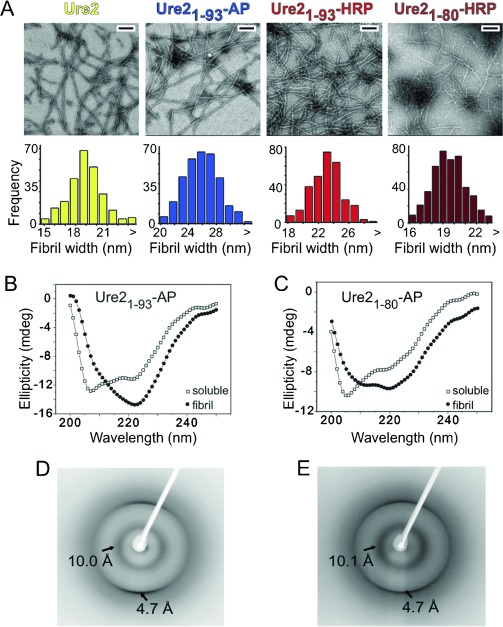
Chimeras between the Ure2 prion domain and AP or HRP enzymes form amyloid fibrils. A) Upper panel: negative-staining TEM of WT Ure2 and chimeric fibrillar aggregates. All scale bars 200 nm. Lower panel: widths of Ure2 and chimeric fibrils measured from TEM images. WT Ure2, Ure21−93-AP, and Ure21−93-HRP show median widths of 19, 26, and 23 nm (n>300), respectively, consistent with their relative molecular weights. Ure21−80-HRP fibrils have a smaller diameter than HRP chimeric fibrils that contain the full-length prion domain of Ure2 (residues 1–93). CD spectra of native (open squares) and fibrillar (closed circles) B) Ure21−93-AP and C) Ure21−80-HRP. Fibril assembly of chimeric proteins is associated with an increase in β-sheet content. X-ray fiber diffraction patterns of D) Ure21−93-AP fibrils and E) Ure21−80-HRP fibrils, which show anisotropic reflections at 4.7 and 10 Å.
