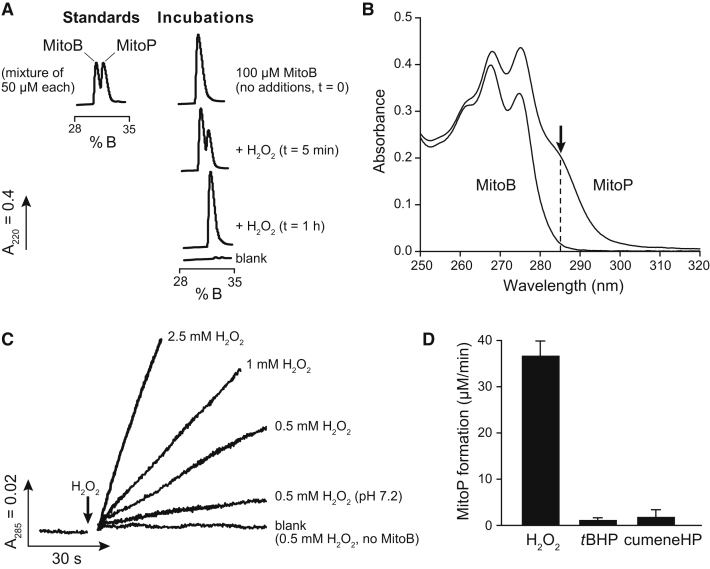
Figure 2.
Reaction of MitoB with H2O2 to Form MitoP
(A) Oxidation of MitoB to MitoP assessed by RP-HPLC. MitoB (100 μM) was incubated at 37°C in KCl medium (pH 8.0) with no additions or with 100 μM H2O2 and then analyzed by RP-HPLC. A mixture of MitoB and MitoP standards (50 μM each) was also analyzed.
(B) Absorbance spectra of MitoB and MitoP (100 μM) in KCl medium, showing a large difference in absorption at 285 nm due to the phenol moiety.
(C) Progress curves for the reaction of MitoB with H2O2. The conversion of MitoB (100 μM) to MitoP by reaction with H2O2 is measured at 285 nm in KCl medium at 37°C (pH 8.0, unless otherwise indicated).
(D) Reaction of MitoB with various peroxides. The conversion rate of MitoB (100 μM) to MitoP by reaction with 500 μM of either H2O2, tert-butylhydroperoxide (tBHP) or cumene hydroperoxide (cumeneHP) was measured at 285 nm in KCl medium at 37°C, pH 8.0. Data are means ± SD, n = 3.