Figure 7. Aurora B inhibition in D458 human medulloblastoma intracranial xenograft model.
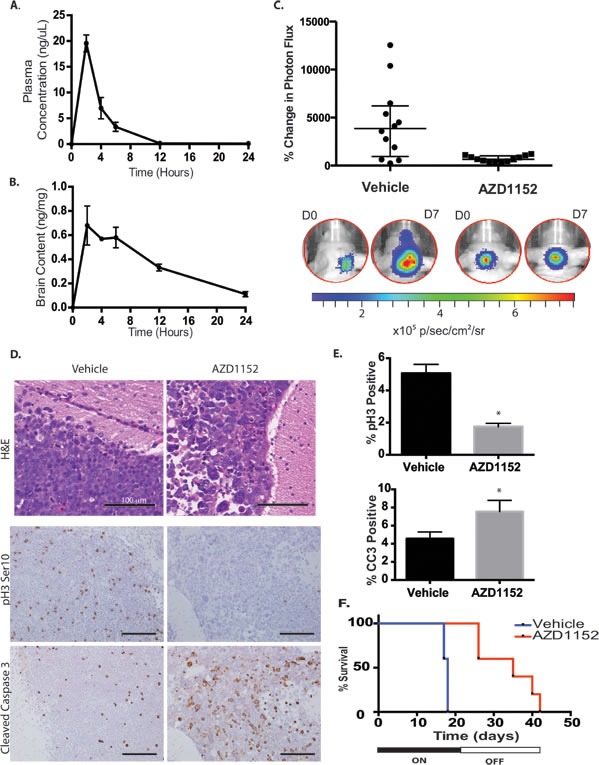
A) Plasma concentration of AZD1152-HQPA in mice measured by LC/MS/MS at different time points after administration of 100 mg/kg subcutaneously in the dorsal skin fold. Error bars represent S.E.M. B) Whole brain content of AZD1152-HQPA in non-tumor bearing mice measured by LC/MS/MS at different time points after subcutaneous administration of 100 mg/kg (~3 mg per animal). Error bars represent S.E.M. C) Bioluminescence measurements represented as a change in photon flux from intracranial tumor at 1 week compared to start of treatment in mice treated with vehicle (n=12) or AZD1152-HQPA 50 mg/kg/day (n=12), P<0.01. D) H&E stain and immunohistochemistry for Histone H3 Serine 10 phosphorylation (pH3 Ser10) and cleaved caspase-3 (CC3) on cerebellar D458 tumors in mice that received either vehicle (n=6) or AZD1152-HQPA 50 mg/kg/day x 7 days (n=7). Scale bars 100 μm. E) Percentage of cells positive for pH3Ser10 or cleaved caspase-3 in cerebellar D458 tumors from mice that received either vehicle (n=6) or AZD1152-HQPA 50 mg/kg/day x 7 days (n=7), *P<0.05. F) Survival curve for mice bearing D458 cerebellar tumors treated with vehicle (n=5) or AZD1152-HQPA (n=5) 50 mg/kg/day for 21 days beginning 7 days after tumor cell implantation, Log-Rank P=0.003.
