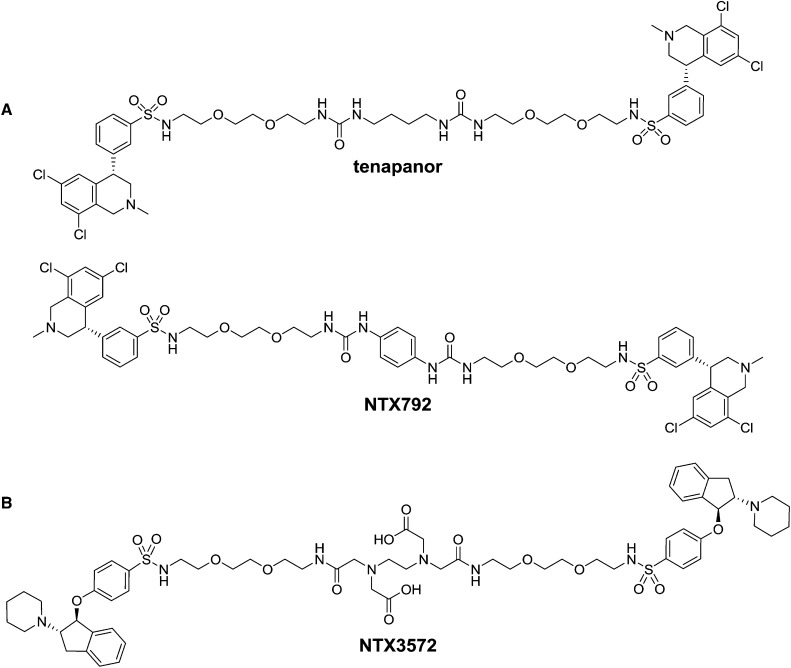
Figure 1.
Structures of NHE3 inhibitors with minimal systemic bioavailability. (A) Shown tetrahydroisoquinoline dimers are tenapanor [(S)-N,N′-(10,17-dioxo-3,6,21,24-tetraoxa-9,11,16,18-tetraazahexacosane-1,26-diyl)bis(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)benzenesulfonamide) dihydrochloride] and NTX792 [(S)-N,N′-(2,2′-(2,2′-(2,2′-(1,4-phenylenebis(azanediyl))bis(oxomethylene)bis(azanediyl)bis(ethane-2,1-diyl))bis(oxy)bis(ethane-2,1-diyl))bis(oxy)bis(ethane-2,1-diyl))bis(3-((S)-6,8-dichloro-2-methyl-1,2,3,4-tetrahydroisoquinolin-4-yl)benzenesulfonamide) dihydrochloride]. (B) Shown indane dimer is NTX3572 [6-(carboxymethyl)-8-oxo-3-(2-oxo-2-(2-(2-(2-(4-((1S,2S)-2-(piperidin-1-yl)-2,3-dihydro-1H-inden-1-yloxy)phenylsulfonamido)ethoxy)ethoxy)ethylamino)ethyl)-17-(4-((1S,2S)-2-(piperidin-1-yl)-2,3-dihydro-1H-inden-1-yloxy)phenylsulfonamido)-12,15-dioxa-3,6,9-triazaheptadecan-1-oic acid].