Abstract
Purpose
To report the ophthalmologic and histologic findings in a series of children with infantile Pompe disease treated with enzyme replacement therapy (ERT).
Methods
We reviewed the records of children with infantile Pompe disease treated with ERT who had at least one complete ophthalmic examination and the ocular histopathology of children with infantile Pompe disease who were treated with ERT. We reviewed the patient’s clinical history, including external ocular examination, ocular alignment and motility, dilated fundus examination, and cycloplegic refraction. A literature review was performed for ophthalmologic findings in infantile Pompe disease using PubMed.
Results
We included the clinical findings of 13 children and reviewed the ocular histopathology of three children with infantile Pompe disease who were treated with ERT. Forty-six percent (6/13) had bilateral ptosis, 23% (3/13) strabismus, 62% myopia (8/13), and 69% (9/13) astigmatism. On histologic examination, there was vacuolar myopathy affecting the extraocular muscles, ciliary body, and iris smooth muscle and glycogen accumulation in corneal endothelial, lens epithelium, and retinal ganglion cells, and within lysosomes of scleral fibroblasts.
Conclusions
It is important that ophthalmic providers are aware of the high prevalence of myopia, astigmatism, and ptosis in children with infantile Pompe disease treated with ERT, as they are potentially amblyogenic, but treatable factors.
Introduction
Pompe disease is an inherited (autosomal recessive) lysosomal storage disorder caused by a deficiency of the enzyme acid alpha-glucosidase (GAA), which results in glycogen accumulation in various body tissues.1 Based on age of onset, organ involvement, and degree of myopathy, Pompe disease is broadly classified into two forms: infantile- and late-onset.2
The infantile form includes those whose symptoms begin before one year of age, and can be divided into two subtypes (classic and atypical), based on the severity and presence/absence of cardiomyopathy.3 Prior to the advent of enzyme replacement therapy (ERT) with alglucosidase alfa, most infantile Pompe patients, in particular those with the classic form (those with severe cardiomyopathy and respiratory failure), did not survive past their first birthday.4 The introduction of ERT has dramatically improved their survival.5
To our knowledge, we are the first to report the ophthalmologic and histologic findings in a series of children with infantile Pompe disease treated with ERT.
Patients and Methods
This study was approved by the Duke Health System Institutional Review Board and was compliant with the requirements of the United States Health Insurance Portability and Accountability Act. Written informed consent was obtained for each subject from the legal guardian. Verbal assent was obtained from all subjects at least 6 and less than 12 years of age. We reviewed the records of 13 children with infantile Pompe disease treated with ERT who had at least one complete ophthalmic examination and the post-mortem specimens of 3 children (one of whom was included in the clinical portion of this study) with infantile Pompe disease who were treated with ERT. Subjects were recruited from cases seen at Duke University Medical Center or from their participation in research studies on Pompe disease at Duke University. All patients had both a clinical (hypotonia and developmental delay in the first year of life) and genetic (GAA enzyme activity less than 1% in skin fibroblasts and 2 severe mutations in the GAA gene (Table 1, online only)) diagnosis of infantile Pompe disease.
Table 1.
Baseline Demographics and Mutations in the acid alpha-glucosidase (GAA) gene in 13 Children with Infantile Pompe Disease Treated with Enzyme Replacement Therapy (ERT)
| Case | Gender | Race | Age at Diagnosis (months) |
Age at first ERT infusion (months) |
Allele 1 cDNA change (amino acid change) |
Allele 2 cDNA change (amino acid change) |
|---|---|---|---|---|---|---|
| Classic Infantile Pompe Cases | ||||||
| 1 | M | Asian Indian |
1 | 3 | c.1933G>A (p.Asp645Asn) | c.1933G>A (p.Asp645Asn) |
| 2 | F | H | 3 | 5 | c.1802C>T (p.Ser601Leu)a | c.1099T>C (p.Trp367Arg) |
| 3 | M | H | 7 | 7 | c.2297A>C (p.Tyr766Ser) | c.2297A>C (p.Tyr766Ser) |
| 4 | M | C | 2 | 2 | c.1933G>A (p.Asp645Asn) | c.1933G>A (p.Asp645Asn) |
| 5 | F | C | 6 | 6 | c.655G>A (p.Gly219Arg) | c.655G>A (p.Gly219Arg) |
| 6 | M | C | 0 | 1 | c.525delT (p.Glu176ArgfsX45) | c.1642G>T (p.Val548Phe)b c.1880C>T (p.Ser627Phe)b |
| 7 | M | AA | 1 | 1 | c.2560C>T (p.Arg854X) | c.2560C>T (p.Arg854X) |
| 8 | M | AA | 6 | 6 | c.1082C>T (p.Pro361Leu) | c.953T>C (p.Met318Thr) |
| 9 | M | C | 1 | 1 | c.307T>G (p.Cys103Gly) | c.2219_2220delTG (p.Val740GlyfsX55) |
| 10 | F | H | 4 | 6 | c.1195-18_2190-20del (p.Asp399ValfsX6) | c.1195-18_2190-20del (p.Asp399ValfsX6) |
| 11 | F | AA | 5 | 6 | c.2560C>T (p.Arg854X) | c.2560C>T (p.Arg854X) |
| Atypical Infantile Pompe Cases | ||||||
| 12 | M | AA | 15 | 16 | c.-32-17_-32-10delins TCCCTGCTGAGCCTCCTACAGGCCTCCCGC (presumably affects splicing) |
c.1447G>A (p.Gly483Arg) |
| 13 | M | C | 8 | 20 | c.525delT (p.Glu176fsX45) | c.-32-13T>G |
AA, African American; C, Caucasian; F, Female; H, Hispanic; M, Male.
This patient is also heterozygous for the GAA pseudodeficiency alleles, p.[Gly576Ser;Glu689Lys.]
Predicted based on PolyPhen-2: http://genetics.bwh.harvard.edu/pph2/
Mutation nomenclature is written to conform to the recommendations of the Human Genome Variation Society (www.hgvs.org).
References for previously published mutations are available from the Pompe disease mutation database (www.pompecenter.nl; Erasmus Medical Center, Rotterdam).
We reviewed the patients’ clinical history, including external ocular examination, ocular alignment and motility, dilated fundus examination, and cycloplegic refraction. For refractive errors, hyperopia was defined as a spherical equivalent (SE) ≥+0.50D; myopia, SE ≤−0.50D; and high myopia, SE ≤−6.00D.
We also performed a literature search in PubMed for English-language only articles (1946–2013), using combinations of the following search terms: acid maltase deficiency, eye, glycogen-storage disease type II, glycogenosis type II, infantile, ocular, Pompe.
Results
Clinical Ophthalmologic findings
Our series of children included 9 (69%) males and 4 (31%) females (Tables 1 and 2, online only). Eleven had classic and 2 had atypical disease. Average age at first eye examination was 3.2 (range:1.3–5.5) years (Table 3, online only). Eighty-five percent (11/13) had more than one eye examination.
Table 2.
Eye Findings in Children with Infantile Pompe Disease Treated with Enzyme Replacement Therapy
| Case | Age* (years) |
Gender | Refractive Error*,^ | Ptosis | Strabismus | Age at first ERT infusion (months) |
Highest ERT dosing* (mg/kg QOW)$ |
||
|---|---|---|---|---|---|---|---|---|---|
| Hyperopia | Myopia | Astigmatism | |||||||
|
Classic Infantile Pompe Cases | |||||||||
| 1 | 8.9 | M | No | No | No | Yes | No | 3 | 20 |
| 2 | 9.0 | F | No | Yes | Yes | Yes | No | 5 | 40 |
| 3 | 9.8 | M | No | Yes | Yes | No | No | 7 | 40 |
| 4 | 7.3 | M | Yes | No | Yes | No | No | 2 | 40 |
| 5 | 6.8 | F | Yes | No | No | No | X(T) | 6 | 40 |
| 6 | 6.2 | M | No | Yes | Yes | Yes | No | 1 | 20 |
| 7 | 5.6 | M | No | Yes | Yes | No | No | 1 | 40 |
| 8 | 4.7 | M | No | Yes | Yes | Yes | X(T) | 6 | 40 |
| 9 | 2.9 | M | No | Yes | No | No | No | 1 | 40 |
| 10 | 3.8 | F | No | Yes | Yes | Yes | X(T) | 6 | 60 |
| 11 | 1.3 | F | No | Yes | Yes | No | No | 6 | 40 |
|
Atypical Infantile Pompe Cases | |||||||||
| 12 | 7.1 | M | Yes | No | Yes | Yes | No | 16 | 20 |
| 13 | 5.3 | M | Yes | No | No | No | No | 20 | 40 |
F, female; M, male; QOW, every other week; X(T), intermittent exotropia; UK, unknown.
Hyperopia, ≥ +0.50D; Myopia ≤ −0.50D; Astigmatism, ≥1.00D.
At last eye exam
Refractive error in the eye with higher refractive error.
All were initially treated with 20mg/kg of alglucosidase alfa QOW per Myozyme package insert.1
Table 3.
Refractive Error Findings in Children with Infantile Pompe Disease Treated with Enzyme Replacement Therapy
| Case | Age at first and last eye exam (years) |
SE at last exam |
ΔSE/year | Astigmatism at last eye exam |
Family History of Myopia* |
|||
|---|---|---|---|---|---|---|---|---|
| OD | OS | OD | OS | OD | OS | |||
|
Classic Infantile Pompe Cases | ||||||||
| 1 | 1.3 / 8.9 | −0.25 | −0.25 | −0.1 | −0.1 | +0.50 | +0.50 | Yes |
| 2 | 4.9 / 9.0 | −0.25 | −0.75 | −0.2 | −0.3 | +3.50 | +2.50 | Yes |
| 3 | 5.5 / 9.8 | −3.15 | −4.25 | −0.1 | −0.2 | +3.25 | +3.50 | Yes |
| 4 | 4.2 / 7.3 | +3.25 | +3.00 | +0.2 | +0.2 | +1.00 | none | Yes |
| 5 | 3.2 / 6.8 | +1.15 | +1.00 | +0.1 | 0.0 | +0.25 | none | No |
| 6 | 2.4 / 6.2 | −1.25 | −1.50 | −0.3 | −0.4 | +3.00 | +3.00 | Yes |
| 7 | 4.2 / 5.6 | −12.25 | −12.25 | −0.8 | −0.2 | +3.50 | +3.50 | Yes |
| 8 | 2.0 / 4.7 | −3.90 | −5.75 | - | - | +2.25 | +1.50 | Yes |
| 9 | 1.4 / 2.9 | −13.00 | −11.50 | −1.3 | −1.3 | none | none | Yes |
| 10 | 1.4 / 3.8 | −0.65 | −0.65 | −1.0 | −1.0 | +3.75 | +3.75 | No |
| 11 | 1.3 | −1.50 | −1.50 | - | - | +2.00 | +2.00 | Yes |
|
Atypical Infantile Pompe Cases | ||||||||
| 12 | 3.8 / 7.1 | +1.25 | +1.15 | +0.2 | +0.1 | +1.00 | +0.75 | Yes |
| 13 | 5.3 | +1.00 | +1.50 | - | - | none | none | Yes |
OD, right eye; OS, left eye; SE, spherical equivalent in diopters.
All with a family history of myopia were < −2.00sph
Of those with classic disease, 45% (5/11) had bilateral ptosis and 27% (3/11) had strabismus (Table 2, online only). Of those with strabismus, all had an intermittent exotropia. Eighteen percent (4/22) of eyes had hyperopia, 68% (15/22) myopia, and 18% (4/22) high myopia (Table 3, online only). Astigmatism of ≥1.00D was detected in 68% (15/22), of ≥2.00D in 59% (13/22), and of ≥3.00D in 41% (9/22) of eyes. All astigmatism of ≥1.00 D was with-the-rule.
Of the two patients with atypical disease, one had bilateral ptosis and neither had strabismus or myopia (Table 2, online only). One-hundred percent (4/4) of eyes had hyperopia and 25% (1/4) of eyes had astigmatism (of ≥1.00D) (Table 3, online only).
All patients were initially started on an intravenous infusion of 20 mg/kg of alglucosidase alfa every other week (QOW) according to the Myozyme package insert instructions.6 Eighty-two percent (9/11) of those with classic and 50% (1/2) with atypical disease were titrated up to ≥40 mg/kg QOW in an attempt to improve their cardiomyopathy and muscle weakness (Table 2, online only).
Histologic findings
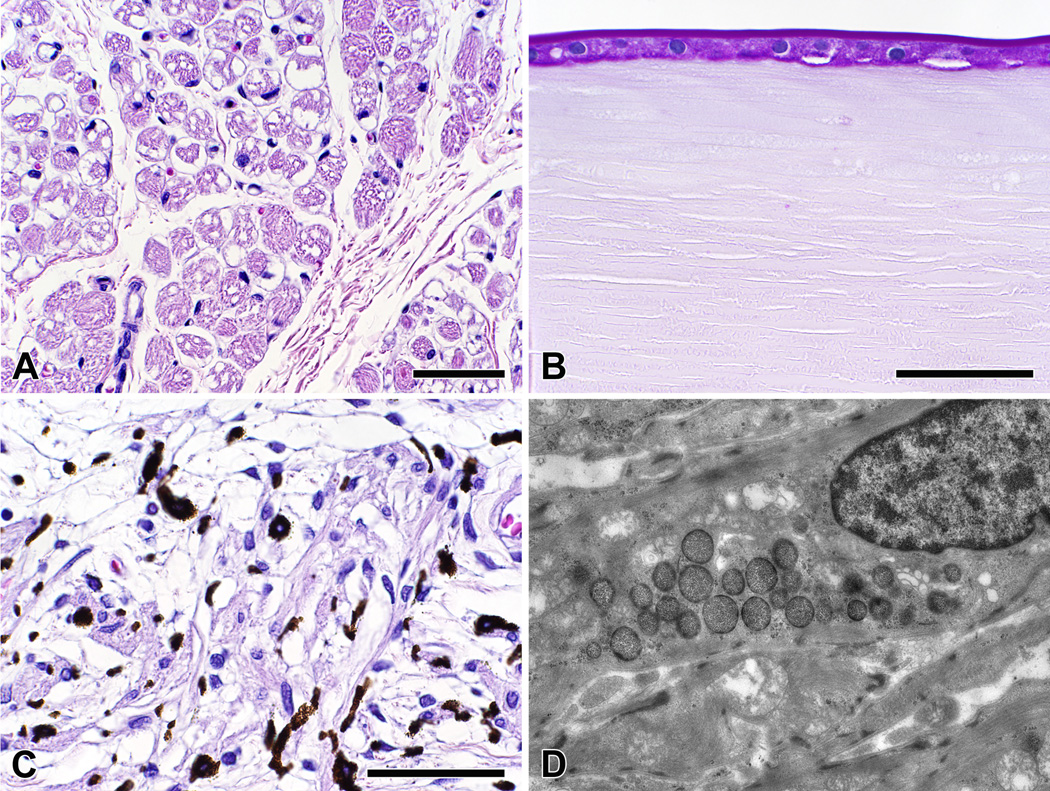
We performed histologic examinations of eyes from a 7-month-old boy treated with ERT for two months, a 1-year-old girl treated with ERT for four months,7 and a 21-month-old girl treated with ERT for 15 months (Case 11). In all cases, there was vacuolar myopathy affecting the extraocular muscle fibers, ciliary body, and iris smooth muscle; moderate accumulation of glycogen in corneal endothelial and retinal ganglion cells; and marked accumulation of glycogen in the lens epithelium (Figure 1A–C). In our previously reported case,7 we also noted glycogen accumulation within lysosomes of scleral fibroblasts (Figure 1D).
Figure 1.
(A) Inferior oblique muscle from a 21-month-old girl (Case 11) treated with enzyme replacement therapy (ERT) for 15 months shows vacuolar myopathy affecting many, but not all, of the extraocular muscle fibers [hematoxylin and eosin, magnification bar = 50 µm]; (B) The lens epithelium from the same child had marked accumulation of glycogen [periodic acid-Schiff reagent, magnification bar = 50 µm]; (C) Ciliary body smooth muscle from this child had marked vacuolar myopathy affecting most of the cells [hematoxylin and eosin, magnification bar = 50 µm]; (D) Transmission electron microscopy of a scleral fibroblast from a 1-year-old girl treated with ERT for 4 months shows glycogen accumulation within lysosomes [original magnification = ×7100].
Discussion
While ophthalmologic findings in infantile Pompe patients have been reported in isolated case reports,7–12 we are the first to report ophthalmologic findings in a series of children with infantile Pompe treated with ERT. Prior to the availability of ERT, two cases of strabismus in a 9-month-old8 and 3-year-old10 boy with Pompe disease had been described. Since the advent of ERT, the presence of not only strabismus,9 but also ptosis9,12 and myopia9 have been reported in infantile Pompe patients who received ERT (Table 4). In addition to the previously reported ophthalmologic findings, we also found a high prevalence of astigmatism in our population of patients.
Table 4.
Previously Reported Eye Findings in Children with Infantile Pompe Disease
| Author, Year | Sex | Age | Type | RE | Ptosis | Strabismus | ERT |
|---|---|---|---|---|---|---|---|
| Toussaint and Danis11, 1964 | F | 5 months | classic | NS | NS | NS | No |
| Goebel et al.8,1978 | M | 9 months | classic | NS | NS | ET | No |
| Yanovitch et al.7, 2010 | F | 1 year | NS | No | No | No | Yes |
| Smith and Reinecke10, 1972 | M | 3 years | NS | NS | NS | V-pattern ET | No |
| Slingerland et al.9, 2010 | M | 4.5 years | classic | High myopia | Yes | NS, had “restricted” extraocular movements | Yes* |
| Yanovitch et al12, 2010 | M | 17 years | atypical | No | Yes | No | Yes^ |
ET, esotropia; ERT, enzyme replacement therapy; F, female; M, male; NS, not specified; RE, refractive error.
Dose not specified.
Initially treated with 20mg/kg of alglucosidase alfa every other week per Myozyme package insert1, which was later increased to 40mg/kg every other week. Within 6 months there was partial resolution of ptosis.
In our series of children with infantile Pompe on ERT, ptosis, strabismus, myopia, and astigmatism were present. About half of our patients had ptosis. While a previous study reported that a child with atypical infantile Pompe disease had partial resolution of ptosis within 6 months of increasing Myozyme from 20mg/kg to 40mg/kg every other week, despite being on the increased dose of Myozyme, one of our patients with severe ptosis and significant chin-up head positioning ultimately required surgical repair (Case 2). The low prevalence of strabismus (n=3, all with classic disease) in our study was surprising considering the significant degree of muscle weakness.
Clinically-significant refractive errors, which we defined as those in which the ophthalmologist prescribed spectacles, were only seen in those with classic disease, due to myopia and astigmatism. About two-thirds of those with classic disease had myopia, of which about a third of those had high myopia. A family history of myopia was not associated with myopia in those with classic disease (p=0.44).
Based on our histological observations, we hypothesize that myopia may be due to one or more of the following: 1) elongation of the globe caused by compression from enlarged and possibly stiffened extraocular muscles due to glycogen accumulation; 2) induced myopia caused by accumulation of glycogen in the lens which may cause osmotic lens swelling in a fashion similar to that in diabetics;13 3) induced myopia caused by glycogen accumulation in ciliary smooth muscle reducing tension on zonules and allowing the lens to become more spherical; and 4) scleral elongation caused by altered viscoelastic properties of the sclera due to the disruption of the normal synthesis of collagen, proteoglycans, and/or non-collagenous glycoproteins due to the accumulation of glycogen within scleral fibroblasts.14 While biometry and keratometry measurements would help clarify if myopia was due to an increase in axial length or changes in the refractive power of the cornea or lens, these measurements were not routinely obtained, which is a limitation to our study. Another study limitation is our small sample size, but we hope our findings will help elucidate the etiologies of ophthalmic findings seen in these patients and help focus future treatment strategies.
Most children with classic disease had astigmatism, and more than half had ≥2.00D of astigmatism. All astigmatism of ≥1.00D was with-the-rule. While ptosis can cause with-the-rule astigmatism, astigmatism was not more common in those with ptosis (p=0.64). Interestingly, our patient who underwent unilateral ptosis repair had a shift in astigmatism from 4.00D to 2.50D after ptosis surgery (Case 2). Thus, while ptosis may have some role in the etiology of with-the rule astigmatism seen in these patients, ptosis is likely not the sole cause of the astigmatism.
In conclusion, our findings suggest that infantile Pompe patients on ERT, in particular classic infantile-onset disease, have a high prevalence of clinically-significant ophthalmic findings, that are potentially amblyogenic, but treatable and merit annual comprehensive ophthalmologic evaluation. Further longitudinal studies are needed to further define the spectrum of ocular abnormalities that may arise as survival rates improve.
Acknowledgments
The authors thank Deeksha Bali and Jennifer Goldstein for their help compiling genetic mutations.
Financial Support:
Dr. Prakalapakorn is supported by NIH EY016333. Drs. Kishnani and Mendelsohn have received research/grant support from Genzyme Corporation (Cambridge, MA). Dr. Kishnani is a member of the Pompe Disease Advisory Board and the Gaucher Registry Advisory Board for Genzyme Corporation, and has received honoraria from Genzyme Corporation.
The funding organizations had no role in the design or conduct of this research or the decision to submit this report for publication.
Footnotes
Meeting Presentations: none
Financial Conflict of Interest:
Drs. Prakalapakorn, Proia, Yanovitch, and Aleck and Stephanie DeArmey have no financial or proprietary interest in the materials presented herein.
References
- 1.Hirshhorn R, Reuser AJJ. Glycogen storage disease type II: acid α-glucosidase (acid maltase) deficiency. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2001. pp. 3389–3420. [Google Scholar]
- 2.Kishnani PS, Steiner RD, Bali D, et al. Pompe disease diagnosis and management guideline. Genet Med. 2006;8:267–288. doi: 10.1097/01.gim.0000218152.87434.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slonim AE, Bulone L, Ritz S, Goldberg T, Chen A, Martiniuk F. Identification of two subtypes of infantile acid maltase deficiency. J Pediatr. 2000;137:283–285. doi: 10.1067/mpd.2000.107112. [DOI] [PubMed] [Google Scholar]
- 4.Kishnani PS, Hwu WL, Mandel H, Nicolino M, Yong F, Corzo D. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148:671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 5.Kishnani PS, Corzo D, Leslie ND, et al. Early treatment with alglucosidase alpha prolongs long-term survival of infants with Pompe disease. Pediatr Res. 2009;66:329–335. doi: 10.1203/PDR.0b013e3181b24e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myozyme package insert. Cambridge, MA: Genzyme Corporation; 2012. [Google Scholar]
- 7.Yanovitch TL, Banugaria SG, Proia AD, Kishnani PS. Clinical and histologic ocular findings in Pompe disease. J Pediatr Ophthalmol Strabismus. 2010;47:34–40. doi: 10.3928/01913913-20100106-08. [DOI] [PubMed] [Google Scholar]
- 8.Goebel HH, Kohlschutter A, Pilz H. Ultrastructural observations on the retina in type II glycogenosis (Pompe's disease) Ophthalmologica. 1978;176:61–68. doi: 10.1159/000308694. [DOI] [PubMed] [Google Scholar]
- 9.Slingerland NW, Polling JR, van Gelder CM, van der Ploeg AT, Bleyen I. Ptosis, extraocular motility disorder, and myopia as features of pompe disease. Orbit. 2011;30:111–113. doi: 10.3109/01676830.2010.546932. [DOI] [PubMed] [Google Scholar]
- 10.Smith RS, Reinecke RD. Electron microscopy of ocular muscle in type II glycogenosis (Pompe's disease) Am J Ophthalmol. 1972;73:965–970. doi: 10.1016/0002-9394(72)90468-0. [DOI] [PubMed] [Google Scholar]
- 11.Toussaint D, Danis P. [Eye Histopathology Study of a Case of Generalized Glycogenosis (Pompe Disease)] Bull Soc Belge Ophtalmol. 1964;137:313–325. [PubMed] [Google Scholar]
- 12.Yanovitch TL, Casey R, Banugaria SG, Kishnani PS. Improvement of bilateral ptosis on higher dose enzyme replacement therapy in Pompe disease. J Neuroophthalmol. 2010;30:165–166. doi: 10.1097/WNO.0b013e3181ce162a. [DOI] [PubMed] [Google Scholar]
- 13.Bron AJ, Sparrow J, Brown NA, Harding JJ, Blakytny R. The lens in diabetes. Eye. 1993;7(Pt 2):260–275. doi: 10.1038/eye.1993.60. [DOI] [PubMed] [Google Scholar]
- 14.Rada JA, Shelton S, Norton TT. The sclera and myopia. Experimental eye research. 2006;82:185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]