Abstract
Recent developments promise to significantly advance the understudied behavioral and neurobiology of aggression: (1) Animal models that capture essential features of human violence and callousness have been developed. These models range from mice that have been selectively bred for short attack latencies, monogamous prairie voles, and glucocorticoid-compromised rats to rodents and non-human primates that escalate their aggression after consuming or when withdrawing from alcohol. (2) Optogenetic stimulation and viral vector-based approaches have begun to identify overlapping and distinctive neural microcircuits and intracellular molecules for adaptive vs. excessive, maladaptive aggressive behavior in several rodent models. Projections from hypothalamic and mesencephalic neurons to the medial prefrontal cortex contain microcircuits that appear pivotal for the escalation of aggression.
Two significant developments during the last decade have enhanced our understanding of the brain mechanisms of excessive aggressive behavior. First, recent advances in preclinical research have led to animal models of aggression that capture the salient features of acts of human violence and callousness [1–4]. Second, novel neurobiological methods such as optogenetics and viral vector-based approaches have begun to identify overlapping and distinctive microcircuits and intracellular molecules for adaptive vs. excessive, maladaptive aggressive behavior in several animal models [5–8].
What is Aggression in Excess?
Ethological studies of aggression focus on the distal and proximal causes, the ontogenetic and phylogenetic origins of aggressive behavior [9]. This framework for adaptive species-typical aggressive behavior allows for the assessment of maladaptive and excessive aggression.
When aggressive behavior escalates to maladaptive levels in rodents [10–12], it is operationally defined by:
Low provocation threshold, short latency to initiate attack;
High rate;
High intensity, leading to significant tissue damage;
Lack of species-normative behavioral structure (i.e., threats are deficient in conveying signaling intentions, and lack of context, critical features of the opponent such as age, sex, or locale are misjudged);
Atypically long aggressive bursts;
Insensitivity to long-term consequences;
Disregard of appeasement signals.
The presently available animal models attain face validity by implementing isomorphic signs and symptoms of excessive aggression, but their phylogenetic and ontogenetic development can only be inferred (i.e., low construct validity).
Animal Models of Maladaptive, Pathological Aggression
(1) Selective breeding and ethological models for escalated aggression
Escalated aggressive behavior with pathological features is evident in mouse and rat strains that are selectively bred for high aggression [1]. Direct comparisons of independent selection experiments identified SAL (short attack latency) mice [13] as the strain displaying the most compelling abnormal and pathological forms of attack [14]. In addition to escalated aggression, SAL mice, derived from wild-trapped rodent colonies, are also characterized by low heart rate, glucocorticoids, brain serotonin levels, and reuptake transporter activity, but elevated serotonin-1A autoreceptor activity relative to other high-aggression mouse lines [15].
The prairie vole (Microtus ochrogaster) has recently emerged as a viable animal model for investigating the neurobiology of escalated aggression and violence [16], using advanced genetic tools to reveal the neural mechanisms mediating maladaptive and excessive agonistic behavior [17]. Ethologically, mating induces intense fatal forms of offensive attack behavior directed toward both male and female conspecifics but not toward their familiar female partner (i.e., selective aggression) in the wild; this can be modeled under well-controlled laboratory conditions [5;18]. In pair-bonded males parvocellular vasopressin neurons in the nucleus circularis and medial supraoptic nucleus are activated during aggression [18] and release their contents in the anterior hypothalamic nucleus, activating vasopressin-V1a-type receptors (V1aRs) to facilitate selective aggression toward novel females but not toward their female partner [5]. Two weeks of sociosexual experience induces structural plasticity of V1aRs to mediate selective aggression, while viral-vector-mediated gene transfer of V1aR into the anterior hypothalamic nucleus, of sexually naïve males recapitulates pair bonding-induced aggression [5]. Furthermore, low dose (1 mg/kg, i.p.) repeated treatment with the psychostimulant d-amphetamine in bonded and unmated males produces vicious attacks toward both familiar and unfamiliar females [5].
The unambiguous qualitatively and quantitatively escalated forms of aggression in a relatively small number of individuals convey face validity to human violence. Dysfunctions in serotonin and neuropeptide neurotransmission in selectively bred and feral aggressive rodents translate well to impulsively aggressive and violent behavior in humans.
(2) Excessive aggression in the hypoglucocorticoid rat model
Hypoarousal during violence as indexed by low glucocorticoid production, heart rate and skin conductance in patients with antisocial personality disorder and conduct disorder can be modeled in adrenalectomized rats that are maintained by low-level glucocorticoid replacement therapy [2;19]. This model captures the “callous-unemotional” hallmark of Conduct Disorder by the display of dysfunctional attack targeting, the absence of social signaling and reduced autonomic activation.
(3) Alcohol-heightened aggression
From a pharmacological perspective, aggressive behavior can be escalated either by low acute alcohol doses or during withdrawal from prolonged exposure to repeated high alcohol doses, presumably based on separate neural mechanisms. Further pharmacological studies of alcohol-escalated aggressive behavior revealed that antagonists of serotonin 1A and 1B receptors, GABAA receptors, glutamate and corticotrophin releasing factor receptor subtypes can exert behaviorally selective anti-aggressive effects in mice, rats, and monkeys [20–29]. Considerably less is known about the neurobiology of escalated aggressive behavior that emerges during withdrawal from prolonged exposure to alcohol.
One hypothesis links the rewarding effects of alcohol and its underlying neural mechanisms to those of aggressive behavior originates from the mescorticolimbic dopaminergic circuit [30–34]. A second hypothesis focuses on the considerable evidence for the anxiolytic effects of alcohol that may reduce the fear of the stranger (i.e., xenophobia) and thereby disinhibit aggressive behavior [11]. Third, the pro-aggressive effects of alcohol may stem from misperceived threatening stimuli [35]. How can animal models best examine the face validity of these alternative hypotheses for better understanding the aggression-escalating effects of alcohol?
Neurobiological Mechanisms of Aggression in Excess
(1) Pharmacological Studies of Monoamines, Glutamate/GABA, and Neuropeptides
One of the most intriguing hypotheses relating the behavioral and neural mechanisms underlying alcohol-heightened aggressive behavior postulates shared neural mechanisms for escalated alcohol consumption and aggression. Considerable evidence suggests that neural activity in mesencephalic-limbic-cortical loops is required for preferring alcohol over other commodities, seeking out the opportunity to self-administer alcohol, working to obtain alcohol, and resisting the negative consequences of alcohol consumption [36–42]. Abundant data identify the intact ascending monoaminergic pathways as necessary for the reinforcing effects of alcohol. Several ionophoric receptors in monoaminergic, GABA-ergic and glutamatergic cells are activated by alcohol at millimolar concentrations [43]. Neuropeptides such as corticotrophin releasing factor, neuropeptide Y and opioid peptides modulate the monoaminergic, GABA-ergic and glutamatergic networks that mediate the reinforcing and rewarding effects of alcohol [42;44].
There is growing support for the hypothesis that the neural mechanisms mediating alcohol’s reinforcing effects overlap or interact with those that are responsible for aggressive and violent acts which in themselves function as reinforcers. Pharmacological antagonism of dopamine D1 and D2 receptors in the nucleus accumbens diminishes the seeking of the opportunity to fight [33;45]. Direct neurochemical assays reveal increased dopamine release in the nucleus accumbens of rats that consume alcohol and subsequently engage in escalated aggressive behavior [46]. It is pausible that a subpopulation of dopamine terminals in the nucleus accumbens originates in the posterior ventral tegmental area and is pivotal for the display of escalated drinking and fighting.
(2) Optogenetic studies
The role of the ventral tegmental area-medial prefrontal cortex microcircuit mediating escalated aggressive behavior has been strengthened by optogenetic studies with high anatomical and temporal resolution [47;48; Box 1; Figure 1].
Box 1.
Optogenetics
Optogenetic techniques enable specific neurons to express light-sensitive proteins (opsin proteins) which in turn activate (channelrhodopsin) or inactivate (halorhodopsin/archaerhodopsin) neurons, either by making transgenic lines or using commercially available viruses in conjunction with the genetic Cre/LoxP recombination system. Optical stimulation of channelrhodopsin-2 (ChR2) expressed in genetically-encoded neurons propagates action potentials and by manipulating light pulse frequency, neuronal activity patterns can transition from excitation to inhibition within milliseconds.
Figure 1.
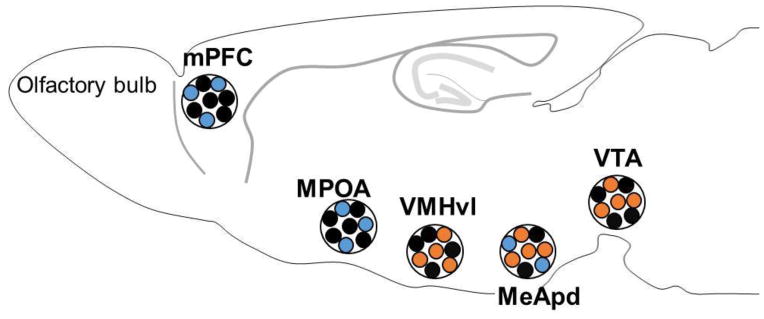
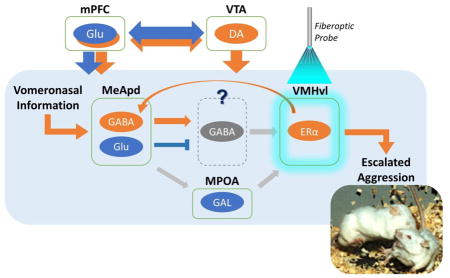
A scheme of brain areas that are involved in inter-male aggression in mice. Brain areas that increase (orange) or decrease (blue) aggressive behavior of male mice when a specific population of neurons within that area was activated by light stimulation. mPFC: medial prefrontal cortex, MPOA: meidal preoptic area, VMHvl: ventrolateral subdivision of ventromedial hypothalamus, MeApd: posterodorsal subdivision of medial amygdala, VTA: ventral tegmental area.
Yu et al. [49] manipulated dopamine and serotonin signaling in the mouse brain and found that reducing monoamine oxidase A during postnatal development (i.e., P2-21), but not in peri-adolescence (i.e., P22-41), enhanced both depression and anxiety whereas monoamine oxidase A blockade during peri-adolescence, but not postnatally or in adulthood (i.e., P182-201), facilitated aggression. Furthermore, reduction of serotonin transporter activity during peri-adolescence blocked aggression. Importantly, reducing activity of the dopamine transporter, but not the norepinephrine transporter, during peri-adolescence similarly enhanced levels of aggression in adulthood. Next, Yu et al. [49] directly stimulated dopaminergic neurons in the ventral tegmental area, which increases dopamine levels in the nucleus accumbens, and found escalated levels of aggression, substantiating prior in vivo microdialysis research in rats that found enhanced dopamine release in the nucleus accumbens during different phases of an aggressive confrontation [50;51]. Transgenic mice expressing the dopamine transporter promoter were crossed with another mouse line containing channelrhodopsin-2 (ChR2, a light sensitive protein) and tested in the isolation-induced aggression paradigm. Compared to single-mutant controls, experimental mice exhibited significantly longer bouts of aggression when dopamine transporter cells were activated in the ventral tegmental area. This suggests that increased dopamine signaling in mesocorticolimbic circuitry escalates aggressive behavior in adult male mice.
The medial prefrontal cortex represents a further key terminal region for ascending monoaminergic pathways, some of which originate in the ventral tegmental area [52]. A recent study by Takahashi et al. [7] investigated the inhibitory role of this cortical area in aggressive behavior using optogenetics to manipulate the activity of medial prefrontal cortex excitatory neurons during aggression. When excitatory neurons were activated with a calcium promoter fused with a light sensitive opsinproteinin the medial prefrontal cortex, but not the orbitofrontal cortex, inter-male aggression in mice was reduced, while inhibition escalated aggression. Conversely, Wang et al. [53] demonstrated that enhancement of glutamatergic AMPA current in the medial prefrontal cortex caused an increase of social rank while inhibition caused a reduction of dominance status. Thus, the medial prefrontal cortex modulates several forms of aggression and functions to maintain a balance between adaptive and maladaptive agonistic behavior.
The medial prefrontal cortex is not the only target of monoaminergic pathways, but also represents a central node in the classic extra-hypothalamic-limbic circuit controlling aggressive behavior [54;55 Figure 2]. Integration of the ventral tegmental area-nucleus accumbens-medial prefrontal cortex circuit with the periaqueductal grey-hypothalamus-amygdala-medial prefrontal cortex pathway will be a challenging task. The first experiments investigating the role of the hypothalamus in the regulation of aggression using optogenetic stimulation focused on the ventrolateral subdivision of the ventral medial hypothalamus. When the light sensitive protein channelrhodopsin-2 was expressed in the ventrolateral subdivision of the ventral medial hypothalamus of male mice, light activation, but not electrical stimulation, in this brain area produced offensive attacks directed toward male, female, and inanimate objects [6], while silencing the ventrolateral subdivision of the ventral medial hypothalamus reduced inter-male aggression. Because they infused a virus encoding the light-sensitive protein ChR2 that infected all neurons surrounding the ventrolateral subdivision of the ventral medial hypothalamus injection site, the specific cell type facilitating aggression remained elusive. Subsequent work by Lee et al. [56] focused on a subset of ventrolateral ventral medial hypothalamic neurons co-expressing the estrogen receptor alpha (ERα) subtype and investigated their specific role underlying male social behavior. They infused a virus carrying the ERα promoter expressing the light sensitive protein (channelrhodopsin-2) into the ventrolateral subdivision of the ventral medial hypothalamus unilaterally (for stimulation) or bilaterally (for inhibition). Optogenetic stimulation of ERα neurons in the ventrolateral subdivision of the ventral medial hypothalamus triggered attack behavior toward both male and female intruders while inhibition suppressed fighting, suggesting that ERα neurons in this area are necessary and sufficient to initiate and terminate bouts of aggression.
Figure 2.
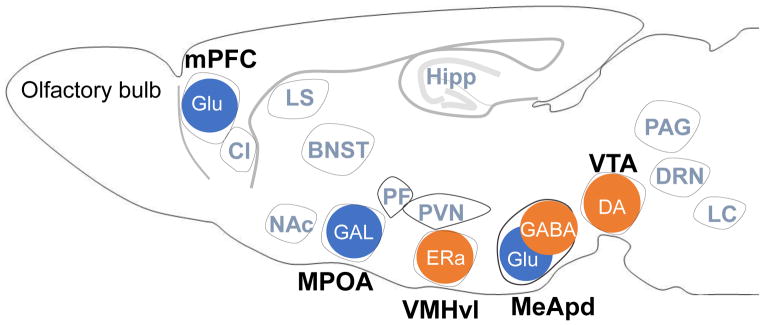
Brain areas that are involved in inter-male aggression in mice. Brain areas that increase (orange) or decrease (blue) aggressive behavior of male mice when a genetically-defined subpopulation of neurons within that area was activated by optogenetic stimulation. Gray colored brain areas are also reported to be involved in aggressive behavior by c-Fos immunohistochemistry (modified from Takahashi et al. [59]). mPFC: medial prefrontal cortex, MPOA: medial preoptic area, VMHvl: ventrolateral subdivision of ventromedial hypothalamus, MeApd: posterodorsal subdivision of medial amygdala, VTA: ventral tegmental area, claustrum (Cl), lateral septum (LS), bed nucleus of the stria terminals (BNST), nucleus accumbens NAcc, piriform cortex (Pir), paraventricular nucleus of the anterior hypothalamus (PVN), parafascicular nucleus of thalamus (PF), hippocampus (Hipp), periaqueductal gray (PAG), serotonin neurons in the dorsal raphe nucleus (DRN), and locus coeruleus (LC). Glu: glutamate, GAL: galanin, DA: dopamine.
The medial preoptic area is another hypothalamic nucleus extensively studied with regard to reproductive and aggressive behavior. In a recent series of elegant experiments, transgenic virgin male mice lacking vomeronasal sensing did not attack pups yet, remarkably, displayed robust paternal behavior [57]. Optogenetic activation of medial preoptic area galanin neurons in virgin males inhibited inter-male and pup-directed aggression but increased offspring grooming. These findings represent the first evidence demonstrating a shift from infanticidal attacks toward offspring to paternal care upon stimulation of galanin neurons in the medial preoptic area.
Afferent and efferent pathways connect the medial amygdala with subdivisions of the hypothalamus to regulate several forms of social behavior. Hong et al. [8] used two transgenic mouse lines to activate glutamatergic or GABA-ergic neurons by injecting a virus expressing the light sensitive protein (ChR2) into the posterior dorsal subdivision of the medial amygdala. Light activation of a subpopulation of GABA-ergic neurons in the posterior dorsal medial amygdala enhanced aggression, while stimulation of nearby glutamatergic neurons increased repetitive self-grooming. These findings reveal an opponent process in which opposing behavioral states are controlled by genetically-defined inhibitory versus excitatory subsets of posterior dorsal medial amygdala cells.
Optogenetic and viral vector-based approaches in rodent models have begun to delineate much more complex microcircuits mediating suppression and escalation of aggressive behavior than suggested by previous methods. Targeting molecularly-defined subtypes of monoaminergic mesocorticolimbic pathways offer novel opportunities for therapeutic intervention. In order to enhance the translational significance of these spatially and temporally high-resolution neurobiological techniques, it will be necessary to incorporate these sophisticated molecular genetic tools in rodent models of escalated aggression.
Highlights.
Violent and callous acts in humans are validly modeled in animals.
These models comprise selective breeding, low glucocorticoids, and alcohol.
Optogenetic stimulation activates microcircuits of escalated aggressive behavior.
These microcircuits encompass the VTA-mPFC and hypothalamic-limbic structures.
Acknowledgments
The preparation of this manuscript and the research from our own laboratory are supported in part by USPHS grants R01-DA031734 and R01-AA013983 (KAM, PI). We thank Dr. J. F. DeBold for discussion and Mr. J.T. Sopko for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Boer SF, Caramaschi D, Natarajan D, Koolhaas JM. The vicious cycle towards violence: focus on the negative feedback mechanisms of brain serotonin neurotransmission. Front Behav Neurosci. 2009;3:52. doi: 10.3389/neuro.08.052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haller J, van de Schraaf J, Kruk MR. Deviant forms of aggression in glucocorticoid hyporeactive rats: a model for ‘pathological’ aggression? J Neuroendocrinol. 2001;13:102–107. doi: 10.1046/j.1365-2826.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- 3.de Almeida RMM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol. 2005;526:51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Miczek KA, Fish EW, de Almeida RMM, Faccidomo S, DeBold JF. Role of alcohol consumption in escalation to violence. Ann N Y Acad Sci. 2004;1036:278–289. doi: 10.1196/annals.1330.018. [DOI] [PubMed] [Google Scholar]
- 5.Gobrogge KL, Liu Y, Young LJ, Wang Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc Natl Acad Sci U S A. 2009;106:19144–19149. doi: 10.1073/pnas.0908620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6**.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. Demonstrated optogenetic, but not electrical, stimulation of neurons in the ventrolateral nucleus of the ventromedial hypothalamus (VMHvl) induced attack toward males, females, and inanimate objects - underscoring the importance of the VMHvl subregion encoding escalated aggression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi A, Nagayasu K, Nishitani N, Kaneko S, Koide T. Control of inter-male aggression by medial prefrontal cortex activation in the mouse. PLoS One. 2014;9:e94657. doi: 10.1371/journal.pone.0094657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Hong W, Kim DW, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158:1348–1361. doi: 10.1016/j.cell.2014.07.049. Found that activation of GABA-ergic neurons in the posterior dorsal medial amygdala (MeApd) enhanced inter-male aggression while glutamatergic stimulation increased self-grooming - illustrating opposing behavioral states controlled via inhibitory and excitatory neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenz K. On Aggression. London: Methuen; 1966. [Google Scholar]
- 10.Haller J, Kruk MR. Normal and abnormal aggression: human disorders and novel laboratory models. Neurosci Biobehav Rev. 2006;30:292–303. doi: 10.1016/j.neubiorev.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Miczek KA, Faccidomo S, de Almeida RMM, Bannai M, Fish EW, DeBold JF. Escalated aggressive behavior: new pharmacotherapeutic approaches and opportunities. Ann N Y Acad Sci. 2004;1036:336–355. doi: 10.1196/annals.1330.021. [DOI] [PubMed] [Google Scholar]
- 12.Miczek KA, de Boer SF, Haller J. Excessive aggression as model of violence: a critical evaluation of current preclinical methods. Psychopharmacology. 2013;226:445–458. doi: 10.1007/s00213-013-3008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Oortmerssen GA, Bakker TCM. Artificial selection for short and long attack latencies in wild Mus musculus domesticus. Behav Genet. 1981;11:115–126. doi: 10.1007/BF01065622. [DOI] [PubMed] [Google Scholar]
- 14.Natarajan D, de Vries H, de Boer SF, Koolhaas JM. Violent phenotype in SAL mice is inflexible and fixed in adulthood. Aggress Behav. 2009;35:430–436. doi: 10.1002/ab.20312. [DOI] [PubMed] [Google Scholar]
- 15.Natarajan D, Caramaschi D. Animal violence demystified. Front Behav Neurosci. 2010;4:9. doi: 10.3389/fnbeh.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobrogge KL. Sex, drugs, and violence: neuromodulation of attachment and conflict in voles. In: Miczek KA, Meyer-Lindenberg A, editors. Neuroscience of Aggression. Heidelberg: Springer; 2014. pp. 229–264. [DOI] [PubMed] [Google Scholar]
- 17.Gobrogge KL, Wang ZW. Genetics of aggression in voles. Adv Genet. 2011;75:121–150. doi: 10.1016/B978-0-12-380858-5.00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- 19.Haller J, Horvath Z, Bakos N. The effect of buspirone on normal and hypoarousal-driven abnormal aggression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:27–31. doi: 10.1016/j.pnpbp.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Weerts EM, Tornatzky W, Miczek KA. Prevention of the proaggressive effects of alcohol by benzodiazepine receptor antagonists in rats and in squirrel monkeys. Psychopharmacology. 1993;111:144–152. doi: 10.1007/BF02245516. [DOI] [PubMed] [Google Scholar]
- 21.Fish EW, Faccidomo S, Miczek KA. Aggression heightened by alcohol or social instigation in mice: reduction by the 5-HT1B receptor agonist CP-94,253. Psychopharmacology. 1999;146:391–399. doi: 10.1007/pl00005484. [DOI] [PubMed] [Google Scholar]
- 22.de Almeida RMM, Rowlett JK, Cook JM, Yin W, Miczek KA. GABAA/α1 receptor agonists and antagonists: effects on species-typical and heightened aggressive behavior after alcohol self-administration in mice. Psychopharmacology. 2004;172:255–263. doi: 10.1007/s00213-003-1661-1. [DOI] [PubMed] [Google Scholar]
- 23.Miczek KA, de Almeida RMM. Oral drug self-administration in the home cage of mice: alcohol- heightened aggression and inhibition by the 5-HT1B agonist anpirtoline. Psychopharmacology. 2001;157:421–429. doi: 10.1007/s002130100831. [DOI] [PubMed] [Google Scholar]
- 24.de Almeida RMM, Miczek KA. Aggression escalated by social instigation or by discontinuation of reinforcement (“frustration”) in mice: inhibition by anpirtoline, a 5-HT1B receptor agonist. Neuropsychopharmacol. 2002;27:171–181. doi: 10.1016/S0893-133X(02)00291-9. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie-Quirk SD, Girasa KA, Allan AM, Miczek KA. 5-HT3 receptors, alcohol and aggressive behavior in mice. Behav Pharmacol. 2005;16:163–170. doi: 10.1097/00008877-200505000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Fish EW, McKenzie-Quirk SD, Bannai M, Miczek KA. 5-HT1B receptor inhibition of alcohol-heightened aggression in mice: comparison to drinking and running. Psychopharmacology. 2008;197:145–156. doi: 10.1007/s00213-007-1017-3. [DOI] [PubMed] [Google Scholar]
- 27.Faccidomo S, Bannai M, Miczek KA. Escalated aggression after alcohol drinking in male mice: dorsal raphe and prefrontal cortex serotonin and 5-HT1B receptors. Neuropsychopharmacology. 2008;33:2888–2899. doi: 10.1038/npp.2008.7. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi A, Kwa C, DeBold JF, Miczek KA. GABAA receptors in the dorsal raphé nucleus of mice: escalation of aggression after alcohol consumption. Psychopharmacology. 2010;211:467–477. doi: 10.1007/s00213-010-1920-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quadros IM, Hwa LS, Shimamoto A, Carlson J, DeBold JF, Miczek KA. Prevention of alcohol-heightened aggression by CRF-R1 antagonists in mice: critical role for DRN-PFC serotonin pathway. Neuropsychopharmacology. 2014;39:2874–2883. doi: 10.1038/npp.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fish EW, DeBold JF, Miczek KA. Repeated alcohol: behavioral sensitization and alcohol-heightened aggression in mice. Psychopharmacology. 2002;160:39–48. doi: 10.1007/s00213-001-0934-9. [DOI] [PubMed] [Google Scholar]
- 31.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 32.Caramaschi D, de Boer SF, de Vries H, Koolhaas JM. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav Brain Res. 2008;189:263–272. doi: 10.1016/j.bbr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- 34.Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- 35.Giancola PR. Alcohol and aggression: theories and mechanisms. In: McMurran M, editor. Alcohol-Related Violence: Prevention and Treatment. John Wiley & Sons; 2013. pp. 37–59. [Google Scholar]
- 36.Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- 37.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- 38.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- 39.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- 40.Barr CS, Newman TK, Lindell S, Becker ML, Shannon C, Champoux M, Suomi SJ, Higley JD. Early experience and sex interact to influence limbic-hypothalamic-pituitary-adrenal-axis function after acute alcohol administration in rhesus macaques (Macaca mulatta) Alcohol Clin Exp Res. 2004;28:1114–1119. doi: 10.1097/01.alc.0000130973.94350.8c. [DOI] [PubMed] [Google Scholar]
- 41.Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li TK, Murphy JM, McBride WJ. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring and Wistar rats. Alcohol Clin Exp Res. 2004;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- 42.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav. 2013;113:20–37. doi: 10.1016/j.pbb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 46.van Erp AM, Miczek KA. Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology. 2007;191:679–688. doi: 10.1007/s00213-006-0637-3. [DOI] [PubMed] [Google Scholar]
- 47.Deisseroth K, Feng G, Majewska AK, Miesenbock G, Ting A, Schnitzer MJ. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liske H, Qian X, Anikeeva P, Deisseroth K, Delp S. Optical control of neuronal excitation and inhibition using a single opsin protein, ChR2. Sci Rep. 2013;3:3110. doi: 10.1038/srep03110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Yu Q, Teixeira CM, Mahadevia D, Huang Y, Balsam D, Mann JJ, Gingrich JA, Ansorge MS. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol Psychiatry. 2014;19:688–698. doi: 10.1038/mp.2014.10. Optogenetic activation of VTA-DA-ergic neurons increased inter-male aggression in mice and developmental manipulation of DA and 5-HT modulated monoaminergic functioning altering aggression and emotional dysfunction in adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Erp AM, Miczek KA. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci. 2000;20:9320–9325. doi: 10.1523/JNEUROSCI.20-24-09320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrari PF, Van Erp AMM, Tornatzky W, Miczek KA. Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci. 2003;17:371–378. doi: 10.1046/j.1460-9568.2003.02447.x. [DOI] [PubMed] [Google Scholar]
- 52.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 53*.Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. Medial prefrontal cortex (mPFC) layer V pyramidal neurons exhibited enhanced excitatory synaptic input relative to subordinate males and changing synaptic efficacy reversed social hierarchy - discovering mPFC neuroplasticity associated with social rank in mice. [DOI] [PubMed] [Google Scholar]
- 54.Hess WR, Brugger M. Das subkortikale Zentrum der affektiven Abwehrreaktion. Helvetica Physiologica Acta. 1943;1:33–52. [Google Scholar]
- 55.Flynn JP. The neural basis of aggression in cats. In: Glass DC, editor. Neurophysiology and Emotion. New York: Rockefeller University Press; 1967. pp. 40–60. [Google Scholar]
- 56**.Lee H, Kim DW, Remedios R, Anthony TE, Chang A, Madisen L, Zeng H, Anderson DJ. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509:627–632. doi: 10.1038/nature13169. Manipulation of VMHvl-ERα neurons facilitated inter-male mouse aggression and laser light manipulation switched excessive fighting to mating. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. Optogenetic activation of MPOA-galanin (GAL) neurons in virgin male mice suppressed inter-male/pup-directed aggression and induced offspring grooming – revealing a neurochemical behavioral switch between ethologically-related forms of aggression and paternal behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi A, Miczek KA. Neurogenetics of aggressive behavior: studies in rodents. In: Miczek KA, Meyer-Lindenberg A, editors. Neuroscience of Aggression. Heidelberg: Springer; 2014. pp. 3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


