Abstract
The helical protein MLKL inserts into cell membranes and forms a permeation pore therein, resulting in cell death. The paper by Su et al. reports that helix 6 regulates the opening of the pore formed by preceding core helices.
Under disease-induced stress, cells launch a suicide protocol that activates formation of a permeation pore in the cell membrane. The pore is formed by the MLKL protein, and allows osmotic swelling and rupture, ultimately leading to cell death. This process is called necroptosis, an emerging form of programmed cell deaths, which is different from its well-known rhyming cousin apoptosis (Sun et al., 2012; Wang et al., 2014). In this issue of Structure, the paper by Su et al. provides critical insights into how MLKL forms the pore in the membrane and, more importantly, how the pore is regulated at a molecular level (Su et al., 2014).
MLKL belongs to a class of proteins which are expressed as soluble polypeptides but insert into the membrane to form permeation pores or channels. They include bacterial toxins such as colicin and diphtheria toxin, as well as the Bak/Bax proteins that play an essential role in apotosis. In MLKL, the N-terminal membrane binding domain (MBD) is connected to the C-terminal regulatory domain whose phosphorylation status regulates the opening of the pore (Su et al., 2014; Wang et al., 2014).
Using NMR spectroscopy, Su et al. found that the MBD of MLKL forms a helical structure in solution, with C-terminal helix 6 sticking out over the top of the four-helix bundle core as if it is a switching lever (Figure 1A). The NMR structure guided the authors to rationally design site-specific fluorescence labeling experiments to map out the membrane binding regions of MBD. They discovered that helix 6 remains in solution while all four helices of the helical core interact with the membrane extensively. These results were sort of anticipated from the loosely packed amphipathic core helices and the overall hydrophilic nature of helix 6 (Su et al., 2014).
Figure 1. A mechanistic model of activation, membrane-binding and pore-formation for necrotoptic MLKL.
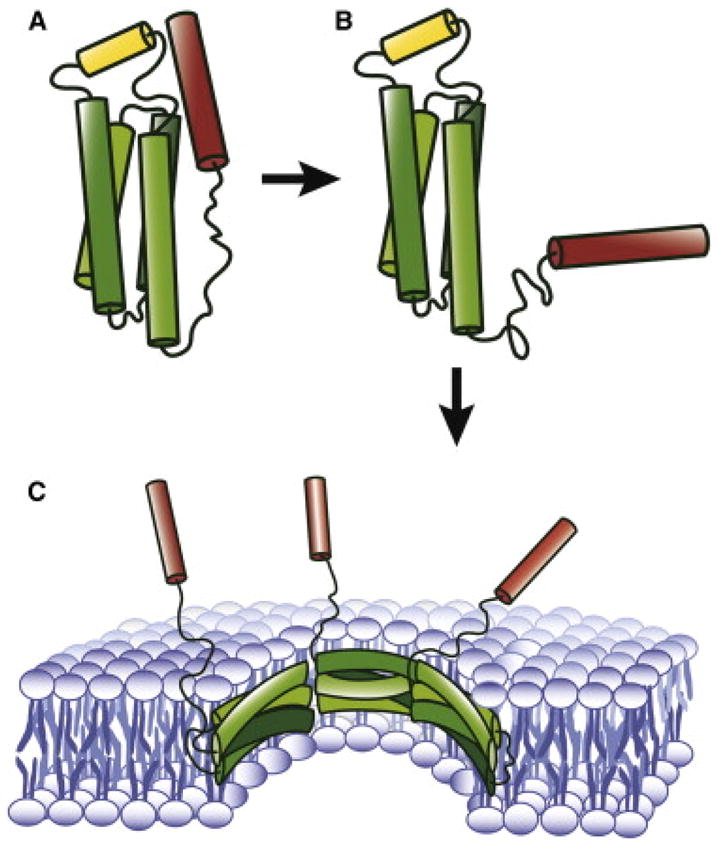
A. Solution structure of MBD. In the native form helix 6 (red) stabilizes the four-helix bundle (green) and inhibits the interaction with the membrane. B. Activated form of MBD. Dissociation of helix 6 from the core, induced by the phosphorylation of the C-terminal domain (not shown) activates MBD to be inserted into the membrane. C. Hypothetical permeable pore formed by MLKL.
Once they established that flanking helix 6 made the direct connection to the regulatory domain, Su et al. wondered if helix 6 might work as an allosteric switch that governs the opening of the pore made of the core helices in the membrane. To test this idea, Su et al. generated a truncation mutant of MBD lacking helix 6 and point mutants that are expected to weaken the interaction between the core and helix 6. Remarkably, these mutants increased vesicle permeability, suggesting that helix 6 functions as the switch for the permeation pore (Su et al., 2014).
In apoptosis, the Bcl-2 family protein Bax (and Bak) also inserts into the mitochondrial outer membrane to form a permeation pore that allows the release of cytochrome c from the intermembrane space to the cytoplasm. This process is considered one of the most critical steps in the mitochondrial pathway of apoptosis. Thus, necroptosis and apoptosis both require membrane permeation.
MLKL may even share a similar mechanism with Bax or Bak for membrane insertion and pore formation. Bax remains folded as an intact soluble protein until it is activated by the binding of the proapoptotic Bcl-2 protein Bid. This binding induces a conformational change, triggering the insertion into the membrane and subsequent formation of an oligomeric pore (Jiang and Wang, 2004). Structurally homologous colicin and DT share the same mechanism although low pH is the trigger for the conformational changes for toxins (Shin et al., 1993). MLKL is structurally somewhat distinct from Bax and toxins. Nonetheless, the dislodging of helix 6 appears to be the prerequisite for membrane binding and pore formation (Figure 1B). Here the phosphorylation of the C-terminal domain by RIP3 seems to trigger the protein conformational change. But the caveats are, (1) MBD binds to membranes containing multivalent anionic lipid cardiolipin or PIP2 spontaneously in vitro (Wang et al., 2014). (2) For aforementioned MLKL mutants, the relative binding to the lipid vesicles were similar or somewhat less compared to wild-type MBD, contrary to our expectations, leaving doors open for the possibility of a different mechanism.
The discoveries by Su et al lead to the next important question, what is the structure of the MLKL pore? The solution structure of MLKL is a mere starting point to answer this question because a large unraveling of the structure is expected to happen upon membrane insertion (Shin et al., 1993). MLKL has the tendency to oligomerize and the resulting oligomeric pore appears to be large enough to allow the passage of molecules bigger than 10kD.
Recently, a glimpse at the architecture of the apoptotic Bak pore has emerged from extensive EPR studies (Aluvila et al., 2014). Here, two Bak molecules were shown to refold into a dimeric amphipathic helical bundle, several of which in turn form a circular amphipathic belt that constitutes the pore. Interestingly here, the membrane-bound helices orient near parallel to the plane of the membrane, just as the helices of apolipoprotein A do when they wrap around the discoidal high density lipoprotein (HDL). Such helical arrangement is divergent from the common architecture of channels and pores formed by integral membrane proteins, where the hydrophobic helices orient largely perpendicular to the membrane surface. Whether the structure of the MLKL pore resembles that of Bak or not remains to be seen. The highly amphipathic nature of the MLKL core helices hints at such possibility (Figure 1C).
Ultimately, the 3-dimensional structure of the MLKL pore will help answer questions about the inner workings of the necrotoptic permeation pore. Nevertheless, the seminal work presented here by Su et al. will stimulate new ideas and experiments concerning structures and functions of membrane-permeation pores by membrane-binding proteins.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 GM051290 (to Y.-K.S.) and the Korea Institute of Science and Technology (KIST Institutional Project #2E25000).
References
- Aluvila S, Mandal T, Hustedt E, Fajer P, Choe JY, Oh KJ. Organization of the mitochondrial apoptotic BAK pore: oligomerization of the BAK homodimers. The Journal of biological chemistry. 2014;289:2537–2551. doi: 10.1074/jbc.M113.526806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annual review of biochemistry. 2004;73:87–106. doi: 10.1146/annurev.biochem.73.011303.073706. [DOI] [PubMed] [Google Scholar]
- Shin YK, Levinthal C, Levinthal F, Hubbell WL. Colicin E1 binding to membranes: time-resolved studies of spin-labeled mutants. Science (New York, NY) 1993;259:960–963. doi: 10.1126/science.8382373. [DOI] [PubMed] [Google Scholar]
- Su L, Quade B, Wang H, Sun L, Wang X, Rizo J. A Plug Release Mechanism for Membrane Permeation by MLKL. Structure. 2014 doi: 10.1016/j.str.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Molecular cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]


