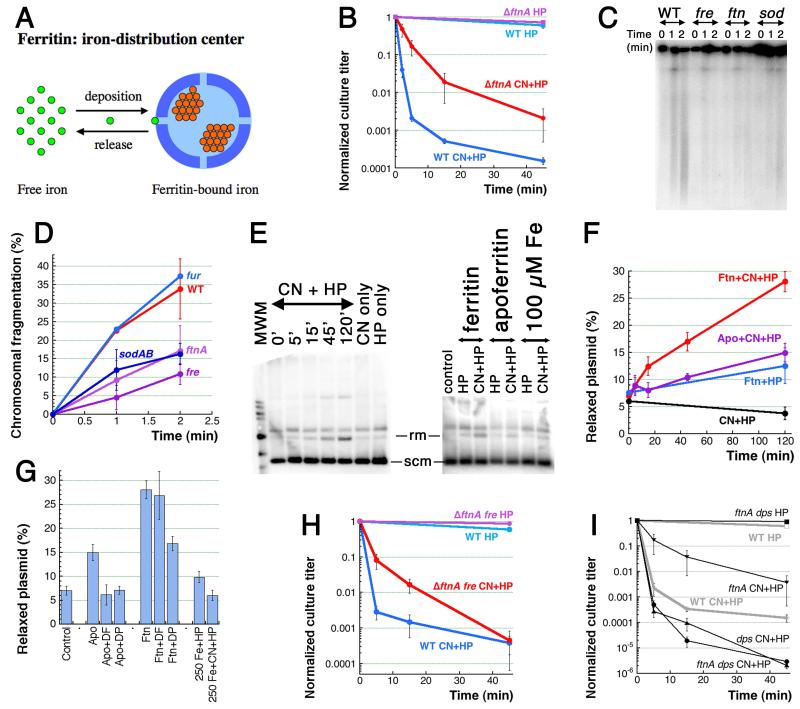
Fig. 6. Ferritin is one source of CN-recruited iron.
A. Ferritin spheres function as iron-distribution centers. Small bright-green circles, Fe(II) iron; small orange circle, Fe(III) iron. B. Kinetics of killing by the two treatments of the ftnA mutant. C. Early chromosomal fragmentation is slower in the ftnA, fre and sodAB mutants — a representative pulsed-field gel of 1′ and 2′ CN+HP treatments. D. Quantification of the early fragmentation from three gels like in “C”. In this case, fragmentation values at 0 time point are subtracted as a background. E. in vitro Fenton reaction with ferritin as a source of iron and plasmid relaxation as a readout. The left panel, kinetics of ferritin + CN + HP, with CN-only and HP-only controls (both at 120 minutes). All reaction have 5 μg of ferritin. The right panel, the apoferritin (also 5 μg, 120 minutes) and the pure iron (the indicated amount, 2 minutes) controls. Rm, relaxed monomer; scm, supercoiled monomer. F. Qauntitative plasmid relaxation kinetics from several gels like in “E”. G. The influence of iron chelators on the plasmid relaxation by ferritin and apoferritin (120 minutes). DF, deferoxamine; DP, dipyridyl. Plasmid relaxation by pure iron in the reaction conditions (treatment length is 2 minutes) is also shown. H. Kinetics of killing by the two treatments of the ftnA fre double mutant. I. Kinetics of killing by the two treatments of the ftnA dps double mutant.