Figure 4. Analysis of P36 peptide cleavage by plasmin in cell culture.
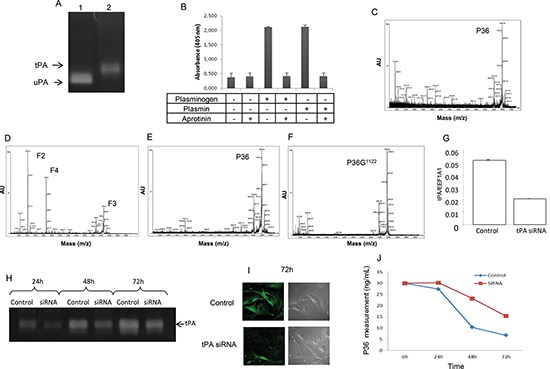
Plasminogen/plasmin activation system in SKMEL28 melanoma cell cultures. (A) Zymography analysis showing that SKMEL28 cells secrete tPA in the extracellular medium (lane 2). No expression of uPA was found. Lane 1 corresponds to uPA positive control. (B) Plasmin activity was measured in the SKMEL28 culture medium using a plasmin Chromogenic substrate S-2251 from Chromogenix, with or without addition of plasminogen and/or aprotinin. (C and D) MALDI-ToF MS analysis of the SKMEL28 culture medium. (C) In the absence of added plasminogen, no cleavage of P36 peptide occurred. (D) In presence of added plasminogen in the cell culture medium, P36 peptide disappeared and F2, F3 and F4 peptides were released by plasmin proteolytic cleavage. (E) When aprotinin was added to the culture medium supplemented with plasminogen, no cleavage of P36 occurred. (F) Substitution of Arg1122 in P36 peptide (P36G1122) suppressed its cleavage by plasmin. SiRNA (tPA) transfection inhibited the cleavage of P36 peptide. (G) Cell transfection with siRNA specific to human tPA decreased tPA mRNA expression. (H) Zymography analysis showing that tPA activity was decreased after siRNA (tPA) transfection. (I) Immunocytofluorescence experiments showing that tPA protein expression at the melanoma cell surface was decreased after siRNA (tPA) transfection. Left panels: immunocytofluorescence data. Right panels: phase contrast microscopy. (J) P36 peptide measurement in the culture medium of SKMEL28 cells transfected with siRNA (tPA). The proteolysis of P36 peptide by plasmin in cell cultures was decreased after siRNA (tPA) transfection.
