Figure 6. Inhibition of xenograft tumor growth by PPIX.
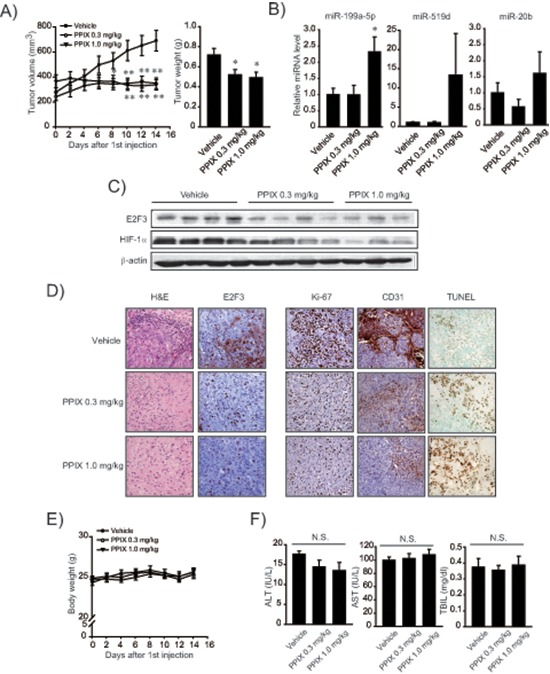
(A) Inhibition of tumor growth. Volumes of tumors originated from SK-Hep1 were measured every other day with vehicle or PPIX treatment as described in the methods section. The mice were sacrificed on day 14 after PPIX treatment, and the excised tumors were weighed. Value represents the mean ± S.E. (n = 8 or 9 in each group, significantly different from vehicle treatment group, *P < 0.05, **P < 0.01, Student's t-test). (B) qRT-PCR assays for miR-199a-5p, -519d, and -20b in xenograft tumors. Tumors obtained as described above were subjected to analyses. Data represents the mean ± S.E (treatment mean significantly different from vehicle-treated control, *P < 0.05). (C) Representative immunoblottings for E2F3 and HIF-1α in the tumor samples. (D) Representative hematoxylin and eosin (H&E) staining, immunohistochemistry for E2F3, Ki-67 and CD31, and TUNEL assays (×200, representative figures were shown; n = 4 in each group). (E) Body weight curves after PPIX treatment at the dose of 0.3 or 1.0 mg/kg body weight for every other day. (F) Alanine aminotransferase (ALT), aspartate aminotransferase (AST) activities, and total bilirubin content (TBIL) in serum after PPIX treatments (N.S., not significant).
