Scheme 1.
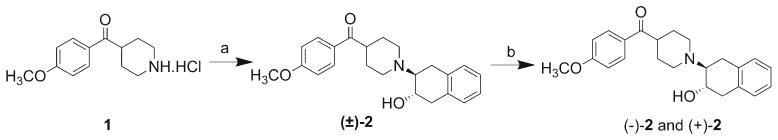
Syntheses of standard compounds (−)-2 and (+)-2. Reagents and conditions: a) 1a,2,7,7a-tetrahydronaphtho[2,3-b]oxirene, K2CO3, EtOH reflux, b) HPLC separation of enantiomers: Chiralcel OD column, mobile phase: 1 % hexane/20 % IPA70% MeOH; flow rate: 4.0 ml/min (+)-2 at 14 min and (−)-2 at 30 min.
