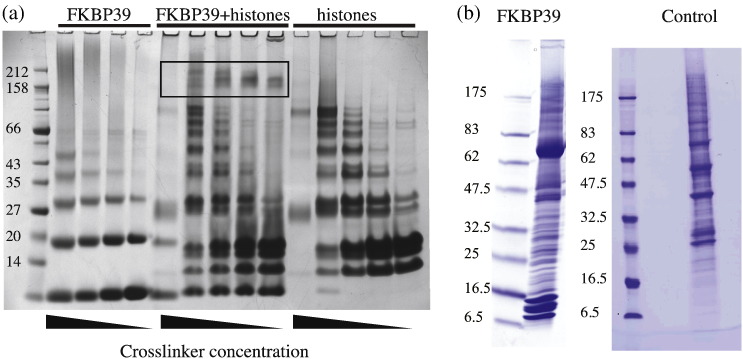
Fig. 6.
Histone binding and pull-down experiments. (a) FKBP39 NPL was cross-linked to a mixture of histones (octamers/dimers/tetramers) with BS3 (Pierce), a reagent that cross-links lysine residues. Both FKBP39 NPL (left) and histones (right) form intra-molecular cross-links, but high-molecular-weight cross-links between FKBP39 and histones can be seen in the range 150–200 kDa. (b) Full-length FKBP39 was tagged at the C-terminus with protein A and expressed in Drosophila D.Mel-2 cells. Cell lysates were bound to IgG beads and washed. The figure shows the eluate from the beads. The bait, FKBP39:protein A, runs at about 70 kDa. The most intense bands are in the low-molecular-weight range and correspond to the four core histones, which appear to be present in stoichiometric amounts. The control is the Drosophila kinetochore protein Nsl1, N-terminally tagged with protein A and purified in the same manner [23]. All gels are Coomassie stained.