Figure 1.
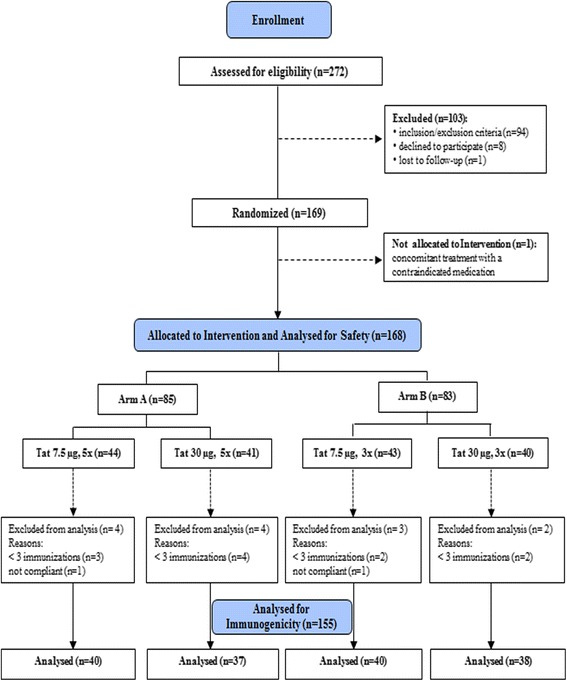
Study flow chart. Two hundred seventy-two HAART-treated patients were assessed for eligibility. One hundred three patients were excluded from the study (n = 94 for either not meeting the inclusion criteria or for meeting exclusion criteria; n = 8 declined to participate, and 1 was lost to follow-up). Thus, 169 subjects were randomized to one of the 4 treatment groups. One subject was excluded after randomization for concomitant treatment with a contraindicated medication, therefore 168 volunteers were allocated to intervention and analyzed for safety (safety population). Since 11 subjects (from the different treatment groups) received less than 3 immunizations and 2 subjects were non compliant with antiretroviral therapy, 155 subjects were considered for the analyses of immunogenicity (immunogenicity population).
