Figure 1.
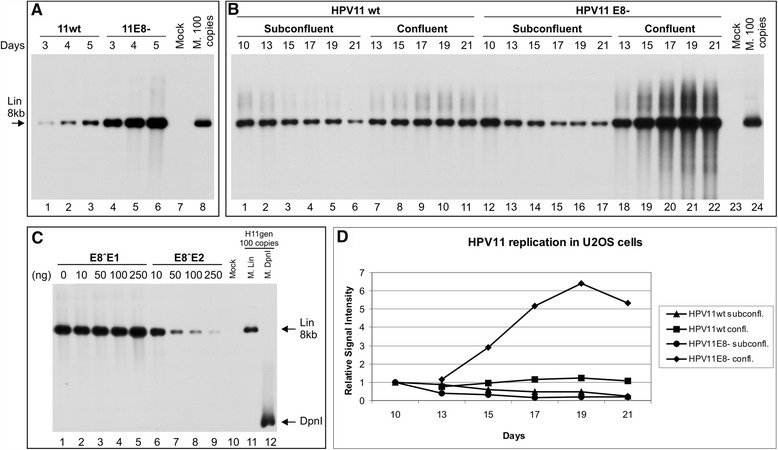
Transient, stable and amplificational replication of the HPV11wt and HPV11E8- genomes in U2OS cells. The mock-transfected cells were used as a negative control (A, lane 7, B, lane 23 and C, lane 10). The linearized HPV11 genome of 100 copies (A, lane 8, B, lane 24 and C, lane 11) and DpnI fragments (C, lane 12) was used as size markers, also indicated with arrows. (A) U2OS cells were transfected with 500 ng of the HPV11wt (lanes 1-3) or E8- (lanes 4-6) genome. Extrachromosomal DNA was extracted via Hirt lysis at 3, 4 and 5 days post-transfection, digested with HindIII and with DpnI. The replication signal was detected via the Southern blot method with a radiolabelled HPV11 genome probe. (B) U2OS cells were transfected with 500 ng of the HPV11wt (lanes 1-11) or HPV11E8- (lanes 12-22) genome, together with 2 μg of the linearized pBabe-Neo construct. The transfected cells were selected with the antibiotic G418, and at 10 days post-transfection, the cells were split and cultivated under either subconfluent (lanes 2-6 and 13-17) or confluent conditions (lanes 7-11 and 18-22). Total DNA was extracted at the time points indicated at top of the figure, and 3 μg of each sample was analyzed as indicated in A. (C) Effect of E8˄E1 and E8˄E2 proteins on viral genome replication. U2OS cells were transfected with 500 ng of the HPV11E8- genome with increasing amounts of either the E8˄E1 or E8˄E2 expression plasmid. Total DNA was extracted at 4 days post-transfection, and 3 μg of each sample was analyzed as indicated in A. (D) The quantitation of HPV11wt and E8- genome DNA replication signals at different time points at subconfluent and confluent culture conditions. The signals were normalized to 10th day time point. Shown is one of two independent experiments.
