Figure 1.
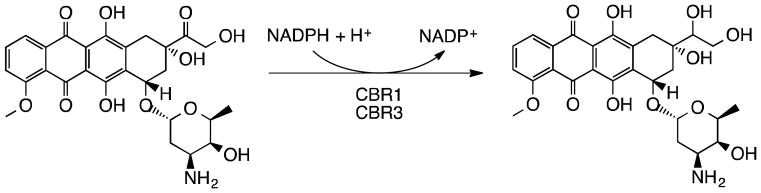
Two-electron reduction of doxorubicin to the putative cardiotoxic alcohol metabolite, doxorubicinol, at the 13th carbon. NADPH-dependent monomeric carbonyl reductase CBR1 is known to mediate this reaction. Here, we demonstrate that this reaction is also carried out by a protein that shares high sequence identity with Cbr1, carbonyl reductase 3.
