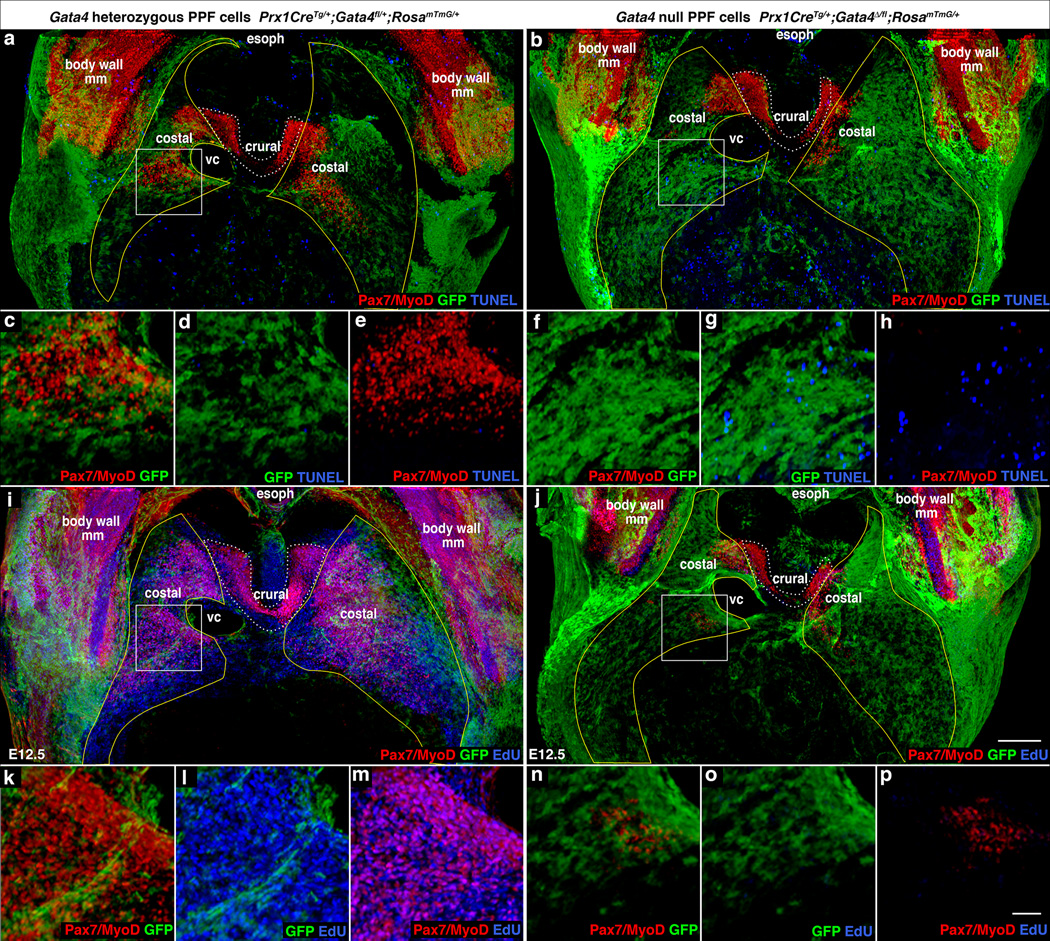
Fig. 6. Early defects in proliferation, apoptosis, and localization of muscle progenitors lead to CDH.
(a–h) At E12.5 there is a marked increase in apoptotic cells in diaphragms with Gata4 null PPF cells (b, f–h versus a, c–e), particularly in the region (boxes a and b, magnified c–e, f–h) that consistently gives rise to hernias (n = 3/3). (i–p) There also is a marked decrease in EdU+ proliferating cells in mutant diaphragms (n = 7/7). (j, n–p versus i, k–m). In the region (boxes i and j, magnified in k–m, n–p) destined to give rise to hernias the few myogenic cells are EdU−. (a–p) Costal muscle progenitors are surrounded by GFP+ PPF cells in control diaphragms but are excluded from regions with GFP+ Gata4 null PPF cells in mutant diaphragms, particularly in destined hernia regions (boxes b, j magnified f–h, n–p). (a–p) Whole-mount immunofluorescence. (a–b, i–j) Pleuroperitoneal folds are outlined in yellow and costal muscle is outlined in white dashed lines. (a–p) Dorsal is at top. Scale bar = 200 µm (a–b, i–j), 50 µm (c–h, k–p). PPF, pleuroperitoneal fold; NT, neural tube; E or esoph, esophagus; So, somite; ST, septum transversum, VC, vena cava, body wall mm, body wall muscles.