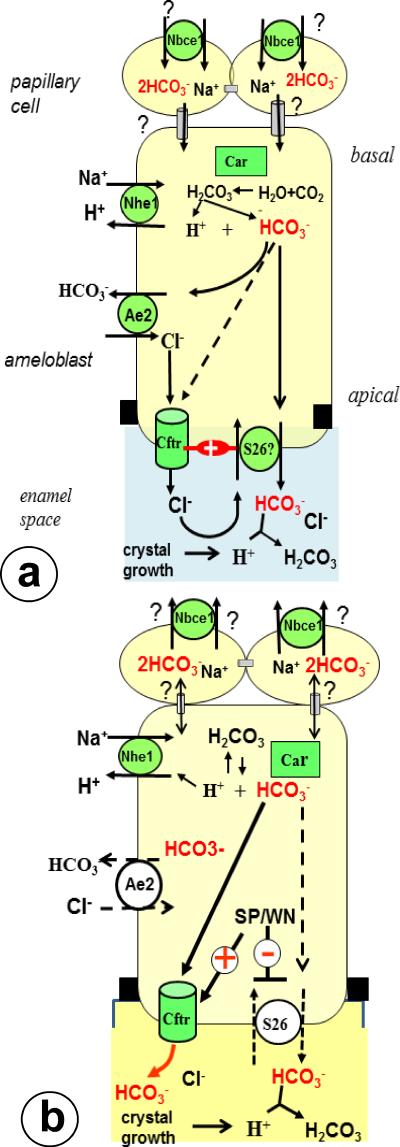
Fig 3.
Simplified work models for buffering of enamel fluid by CFTR in maturation ameloblasts.
Fig 3a. Electroneutral secretion by CFTR and putative anion exchangers. CFTR transfers chloride to drive bicarbonate secretion by apical anion exchangers (potentially the SLC26A family, reference 36). Basolateral AE2 imports chloride that is conduced into enamel by CFTR. A minor part of chloride adsorbs to crystals in the enamel compartment, a major part is exchanged back into the cell for secretion of HCO3-. Putative apical anion exchangers depend on functional CFTR and both stimulate each others activity (“+””). CFTR itself can also transduce small amounts of bicarbonate (about 20%; stippled arrow; ref 22). NBCE1 in papillary layer may import bicarbonate and transfer it through gap junctions into ameloblasts.
Fig 3b. At low intracellular Cl- the CFTR channel switches from an electroneutral predominantly Cl conductive to an electrogenic HCO3- conductive channel activated by WNK, SPAK and OSR1 kinases.
These kinases simultaneously inhibit apical anion exchangers to prevent reabsorption of bicarbonate from the enamel space (reference 37,38). Black squares: tight junctions, gray tubes; gap junctions; Anion exchanger-2; Car carbonic anhydrase 2; NBCE1: Na Bicarbonate Cotransporter-1; NHE1 NaHydrogen Exchanger-1; S26: putative members of SLC26A family; SP Sterile 20-like kinase-related proline/alanine rich kinase (SPAK); WN with-no-lysine kinase or WNK. Not depicted: Na/K ATPase, Carbonic anhydrase 6.