Abstract
PURPOSE
This study aimed to investigate the efficacy of cleaning solutions on saliva-contaminated zirconia in comparison to air-abrasion in terms of resin bonding.
MATERIALS AND METHODS
For saliva-contaminated airabraded zirconia, seven cleaning methods)-no contamination (NC), water-spray rinsing (WS), additional airabrasion (AA), and cleaning with four solutions (Ivoclean [IC]; 1.0 wt% sodium dodecyl sulfate [SDS], 1.0 wt% hydrogen peroxide [HP], and 1.0 wt% sodium hypochlorite [SHC])-were tested. The zirconia surfaces for each group were characterized using various analytical techniques. Three bonded resin (Panavia F 2.0) cylinders (bonding area: 4.5 mm2) were made on one zirconia disk specimen using the Ultradent jig method [four disks (12 cylinders)/group; a total of 28 disks]. After 5,000 thermocycling, all specimens were subjected to a shear bond strength test with a crosshead speed of 1.0 mm/minute. The fractured surfaces were observed using an optical and scanning electron microscope (SEM).
RESULTS
Contact angle measurements showed that groups NC, AA, IC, and SHC had hydrophilic surfaces. The X-ray photoelectron spectroscopy (XPS) analysis showed similar elemental distributions between group AA and groups IC and SHC. Groups IC and SHC showed statistically similar bond strengths to groups NC and AA (P>.05), but not groups SDS and HP (P<.05). For groups WS, SDS, and HP, blister-like bubble formations were observed on the surfaces under SEM.
CONCLUSION
Within the limitations of this in vitro study, some of the cleaning solutions (IC or SHC) were effective in removing saliva contamination and enhancing the resin bond strength.
Keywords: Zirconia, Saliva, Cleaning agent, Dental bonding
INTRODUCTION
Recently, yttria partially stabilized tetragonal zirconia (Y-TZP) has come into wide clinical use mainly due to its high fracture strength.1,2 Although zirconia ceramic restorations may be luted using conventional luting cements,2 bonding with resin to the ceramic would be advantageous for many clinical applications.3 Wegner and Kern3 demonstrated that a durable bond to zirconia was achieved by applying resin luting cements containing 10-methacryloyloxydecyl dihydrogenphosphate (10-MDP) to an air-abraded zirconia surface.
Resin-ceramic bonding might be compromised in clinical situations when compared with clean laboratory situations.1 After the try-in of all-ceramic restoration, the ceramic surface might be contaminated by saliva, blood, or silicone fit-indicators.4 Among them, saliva contamination is reportedly the main cause of decreased resin bond strength.1,5 Some previous studies demonstrated that saliva contamination significantly affected the strength and durability of resin bonds to zirconia and that air-abrasion was the most useful cleaning method.1,4,5,6
Recently, a commercial cleaning solution (Ivoclean, Ivoclar Vivadent, Schaan, Liechtenstein) has been introduced to the dental market. The manufacturer claims that a simple application of the solution, followed by water rinsing and air-drying, effectively cleans the saliva-contaminated bonding surfaces of various dental restorations including zirconia ceramic. However, little research has been carried out with respect to the cleaning efficacy of such cleaning solutions on saliva-contaminated zirconia in terms of resinzirconia bonding.
In this in vitro study, we tested the cleaning efficacy of four (one commercial and three experimental) cleaning solutions in enhancing resin-zirconia bonding following simulation of try-in with saliva exposure and compared it to that of air-abrasion. The hypothesis tested was that the cleaning solutions are less effective than air-abrasion in removing saliva contaminants from zirconia surfaces with respect to zirconia bonding with a 10-MDP-containing resin cement.
MATERIALS AND METHODS
Saliva was collected from one non-smoking male who had refrained from eating and drinking 1.5 hours before the collection procedure, in accordance with the Institutional Review Board of Kyungpook National University Hospital (BMRI 74005-452) and with the informed consent of the donor.1,6 All experiments were performed with fresh saliva.1 Ivoclean (IC, lot #: R78201), which contains zirconium oxide, water, polyethylene glycol, sodium hydroxide, pigments, and additives, was tested. Sodium dodecyl sulfate (SDS), hydrogen peroxide (HP, H2O2), and sodium hypochlorite (SHC, NaOCl) were purchased from Bio-Rad (Richmond, CA, USA; lot #: L1610301) or Duksan Pure Chemicals (Seoul, Korea; lot #: lC8EB41 and 032516, respectively) and diluted with distilled water into 1.0 wt% solutions. The solution codes were likewise used for designating each group in this study.
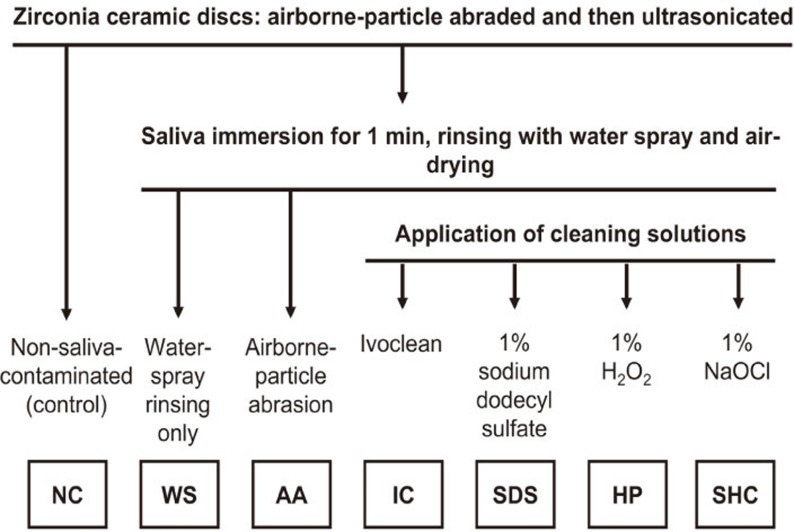
Zirconia (Lava, 3M ESPE, Seefeld, Germany) disk specimens (20 mm diameter and 1.5 mm thickness) were fabricated according to the manufacturer's instructions. Initially, one surface of all specimens was polished with 600 grit silicon carbide (SiC) paper, air-abraded with 50 µm Al2O3 at 0.25 MPa for 15 seconds at a distance of 10 mm,4 ultrasonically cleaned in isopropyl alcohol for 3 minutes, rinsed with water, and finally air-dried.2,7 The specimens were classified into seven study groups. Except for the control group (group NC, no saliva contamination), all specimens were immersed in saliva for 1 minute and rinsed with water-spray for 15 seconds and air-dried for another 15 seconds.4 In group WS, no further cleaning was performed. In group AA, specimens were air-abraded, ultrasonicated, rinsed, and air-dried as described above. In group IC, a microbrush was used to apply IC, which was allowed to react for 20 seconds, followed by rinsing with water-spraying for 15 seconds and air-drying for another 15 seconds, according to the manufacturer's recommendation. In groups SDS, HP, and SHC, the corresponding solutions were applied, respectively, rinsed, and air-dried in the same way as for group IC. The study design for surface analysis and shear bond strength testing is illustrated in Fig. 1.
Fig. 1. Design of this study. All specimens were water-rinsed and finally air-dried prior to further procedures.
To assess the cleanability of the surfaces for each study group,8 water contact angle (CA) measurements were performed. Since roughness may also alter the CA values, surface roughness measurements were performed prior to CA analysis.9,10 The surface roughness Ra of each specimen was measured using a profilometer (Surftest SV-400, Mitutoyo Corp., Kawasaki, Japan) at a stylus speed of 0.1 mm/second, a cutoff of 0.8 mm, and a range of 600 µm.2 The Ra of each specimen was determined as the average of five readings (n=5/group). The CA measurements were performed using a CA goniometer (OCA 15 plus, Data Physics Instrument GmbH, Filderstadt, Germany) in a temperature-controlled room at 23 ± 1℃ with relative humidity at 50 ± 5%.11 Using the dynamic sessile drop method, the advancing CA of water was measured after settling 6 µL droplets on the material surface, the receding one then being measured after sucking 2 µL from the droplet into the syringe (n=5/group).9,12,13 The CA hysteresis (H) was calculated using the equation8: H = cosΘr - cosΘa, in which Θr and Θa are the receding and advancing water CAs, respectively.14 The degree of correlation between the cosΘa and the H was determined by the Pearson correlation coefficient.
To determine the effectiveness of the cleaning methods, specimens of the seven test groups were examined with X-ray photoelectron spectroscopy (XPS).1,6,7 All measurements were performed using an XPS system (PHI Quantera SXM, ULVAC-PHI Inc., Tokyo, Japan) with an X-ray source providing Al Kα X-rays and kinetic energy of 1486.6 eV.1 The emission angle of the photoelectrons was kept constant at 45°. A 180° hemispherical analyzer with 32 channel detectors was used for detection of the photoelectrons.7 A wide scan survey spectrum (0-1100 eV) was obtained to examine the surface composition of the specimens under ultra high vacuum at 10-7 Pa.7 High resolution scans of the carbon (C1s), oxygen (O1s), zirconium (Zr3d), nitrogen (N1s), and aluminum (Al2p) peaks were obtained.1,5 Ratios of C/O, C/Zr, O/Zr, N/Zr, and Al/Zr were calculated.
For shear bond strength testing, a total of 28 zirconia disks were prepared and embedded in round silicone rubber molds using an acrylic resin. The uncovered (to be bonded) surfaces were treated according the study design (Fig. 1) and isolated using a bonding jig (Ultradent Products Inc., South Jordan, UT, USA).9,15 Freshly-mixed Panavia F 2.0 (Kuraray Noritake Dental Inc., Okayama, Japan; lot #: 00586D (A paste), 00114D (B paste, light shade)) was applied to the surface by packing the material into cylindrical-shaped plastic matrices with an internal diameter of 2.38 mm and then irradiated for 20 seconds using a halogen curing light (Elipar TriLight, 3M ESPE; output intensity=750 mW/cm2).9 In this manner, three bonded resin cylinders were made on one zirconia disk specimen and a total of 12 resin cylinders (i.e., four disk specimens) prepared for each group. Prior to debonding, all bonded specimens were stored in water at 37℃ for 24 hours and then thermocycled 5000 times between 5℃ and 55℃ water baths with a dwell time of 30 seconds and a transfer time of 5 seconds between each bath.16
The specimens were perpendicularly engaged at their bonded resin cylinder bases with a round-notched custom shear blade in a universal testing machine (Model 3343, Instron Inc., Canton, MA, USA) at a crosshead speed of 1.0 mm/minute until bonding failure occurred.9,15 Bond strengths (MPa) were calculated from the peak load of failure (N) divided by the bonded surface area. Following debonding, all fractured interfaces were examined under an optical microscope (SMZ800, Nikon Corp., Tokyo, Japan) at 10× magnification to determine the failure mode: A, adhesive failure at the zirconia-resin interface; C, cohesive failure within resin; and M, a combination of these failure modes (mixed failure). In addition, a scanning electron microscope (SEM, JSM-6700F, Jeol, Japan) operating at 5 kV was used to observe the debonded zirconia surfaces.
The Shapiro-Wilks normality test and Levene's variance homogeneity test were applied to the surface roughness and bond strength data. The surface roughness data, which met both the normality and variance homogeneity assumptions, were analyzed using one-way ANOVA. As the bond strength data were normally distributed but showed inhomogeneity of variances between groups, they underwent a log10 transformation to meet homogeneity of variance prior to analysis (Leven's test, P=.135).14 Shear bond strength comparisons between the seven test groups were conducted using one-way ANOVA followed by the Bonferroni post hoc test. The analyses were done under an assumption of independence among the three resin cylinders bonded to each zirconia disk specimen (ST 1).17 In addition, a simple random effect in mixed model ANOVA was conducted to allow correlation between the resin cylinders (ST 2).17 Statistical analyses were carried out using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant at P≤.05 (marginally significant at P≤.1, highly significant at P≤.01, and extremely significant at P<.001).18 In addition, a post hoc power analysis was carried out to examine the power of the bond strength data using G*Power 3.1.7 software.
RESULTS
Table 1 summarizes the surface roughness values of the zirconia specimens. One-way ANOVA revealed no significant differences among the seven groups tested (P=.683), indicating that the additional air-abrasion cleaning (group AA) did not significantly increase the Ra value of the primary air-abrasion (group NC).
Table 1. Ra surface roughness (µm) of the zirconia specimens (mean ± SD, n=5).
| Groups | Ra |
|---|---|
| NC (control) | 0.16 ± 0.02 |
| WS (water-spray) | 0.17 ± 0.02 |
| AA (air-abrasion) | 0.17 ± 0.02 |
| IC (Ivoclean) | 0.17 ± 0.01 |
| SDS (sodium dodecyl sulfate) | 0.17 ± 0.02 |
| HP (H2O2) | 0.17 ± 0.03 |
| SHC (NaOCl) | 0.16 ± 0.01 |
There were no significant differences among the test groups (one-way ANOVA, P=.683).
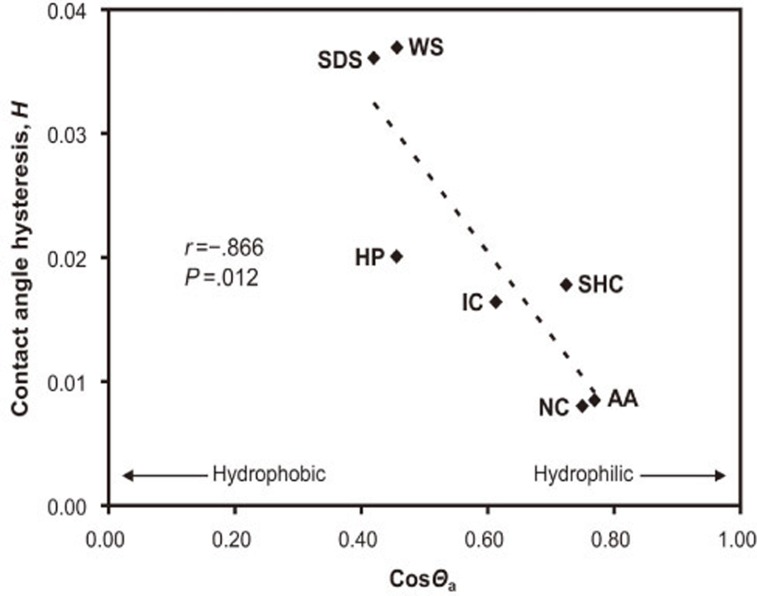
Fig. 2 shows the results of the Pearson correlation analysis between the cosine values of the advancing CAs (cosΘa) and the contact angle hysteresis (H). Groups NC, AA, IC, and SHC showed higher cosΘa values, whereas groups WS, SDS, and HP yielded lower values. A significant strong negative correlation was found between the parameters (r=-.866, P=.012).
Fig. 2. Pearson's correlations between the cosΘa and the contact angle hysteresis (H). r indicates the Pearson's correlation coefficient. AA: air-abrasion; HP: H2O2; IC: Ivoclean; NC: control; SDS: sodium dodecyl sulfate; SHC: NaOCl; WS: water-spray.
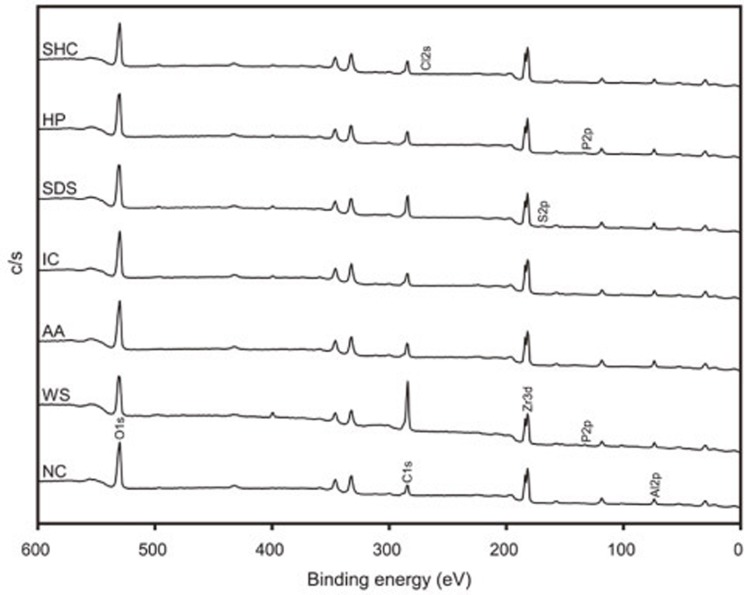
The results of the XPS analysis are shown in Fig. 3 and Table 2. The peak intensity ratios of C/O and C/Zr were the highest in group WS (2.1 and 11.6, respectively). The ratios were reduced after air-abrasion (group AA), being comparable to those of group NC. The four cleaning solution groups showed C/O and C/Zr ratios that were similar to group AA-except for group SDS, which exhibited notably higher ratios. The N element was not detected only in groups NC and AA. In group WS and HP, the phosphorus (P) element was also detected (0.5 and 0.9 at%, respectively). In group SDS, 0.7 at% sulfur (S) element was also detected. On the other hand, group SHC showed 0.5 at% chlorine (Cl).
Fig. 3. Wide-scan XPS spectra for the test groups. AA: air-abrasion; HP: H2O2; IC: Ivoclean; NC: control; SDS: sodium dodecyl sulfate; SHC: NaOCl; WS: water-spray. Al: aluminum; C: carbon; Cl: chlorine; O: oxygen; P: phosphorus; S: sulfur; Zr: zirconium. The peaks between 600 and 0 eV are shown.
Table 2. Ratios of carbon (C), oxygen (O), nitrogen (N), and aluminum (Al) elements.
| Groups | C/O | C/Zr | O/Zr | N/Zr | Al/Zr |
|---|---|---|---|---|---|
| NC (control) | 0.5 | 2.7 | 5.4 | - | 1.2 |
| WS (water-spray) | 2.1 | 11.6 | 5.6 | 0.7 | 1.1 |
| AA (air-abrasion) | 0.6 | 3.4 | 5.9 | - | 1.4 |
| IC (Ivoclean) | 0.6 | 3.1 | 5.0 | 0.2 | 1.0 |
| SDS (sodium dodecyl sulfate) | 0.9 | 5.6 | 5.9 | 0.3 | 1.3 |
| HP (H2O2) | 0.6 | 3.5 | 5.7 | 0.2 | 1.2 |
| SHC (NaOCl) | 0.7 | 3.2 | 4.8 | 0.2 | 0.9 |
Table 3 summarizes the shear bond strength and failure mode results. Each P value from post hoc comparison Bonferroni's test is presented in Table 4. Group SHC showed the highest shear bond strength value (10.9 ± 1.7 MPa). Groups NC, AA, IC exhibited statistically similar bond strength values to that of group SHC. In contrast, groups SDS, WS, and HP showed significantly lower bond strengths than the aforementioned four groups (i.e., groups SHC, NC, AA, and IC). For groups SHC, NC, AA, and IC, mixed failures outnumbered adhesive failures. For groups SDS and HP, the higher frequency of adhesive failures observed when compared to mixed failures. For group WS, all failures were adhesive.
Table 3. Shear bond strength (MPa) of the test groups and type of failure mode.
| Groups | Shear bond strength | Failure modes | |
|---|---|---|---|
| Mean ± SD | A | M | |
| SHC (NaOCl) | 10.9 ± 1.7a* | 4† | 8 |
| NC (control) | 10.4 ± 2.1a | 5 | 7 |
| AA (air-abrasion) | 9.5 ± 2.4a | 6 | 6 |
| IC (Ivoclean) | 9.1 ± 1.3a | 5 | 7 |
| SDS (sodium dodecyl sulfate) | 6.7 ± 1.6b | 8 | 4 |
| WS (water-spray) | 5.7 ± 1.2bc | 12 | 0 |
| HP (H2O2) | 4.4 ± 0.8c | 10 | 2 |
*Means with same lowercase letters are not statistically different at P>.05. The P values are shown in Table 4.
†Number of resin cylinders. A: adhesive failure at the zirconia-resin interface; M: a combination of adhesive failure at the interface and cohesive failure within resin.
Table 4. The P values of the shear bond strength data from post hoc comparison Bonferroni's test.
| Statistics | Groups | WS | AA | IC | SDS | HP | SHC |
|---|---|---|---|---|---|---|---|
| ST 1 | NC | <.001 | >.999 | >.999 | <.001 | <.001 | >.999 |
| WS | <.001 | <.001 | >.999 | .098 | <.001 | ||
| AA | >.999 | .002 | <.001 | >.999 | |||
| IC | .004 | <.001 | .760 | ||||
| SDS | <.001 | <.001 | |||||
| HP | <.001 | ||||||
| ST 2 | NC | <.001 | >.999 | >.999 | .002 | <.001 | >.999 |
| WS | .001 | .001 | >.999 | .331 | <.001 | ||
| AA | >.999 | .030 | <.001 | >.999 | |||
| IC | .043 | <.001 | >.999 | ||||
| SDS | .007 | <.001 | |||||
| HP | <.001 |
ST 1: the analyses were done under the assumption of independence among the three resin cylinders bonded to each zirconia disk specimen. ST 2: a simple random effect in mixed model ANOVA was conducted to allow correlation between the resin cylinders. Means were log10 (MPa) transformed prior to analysis. AA: air-abrasion; HP: H2O2; IC: Ivoclean; NC: control; SDS: sodium dodecyl sulfate; SHC: NaOCl; WS: water-spray.
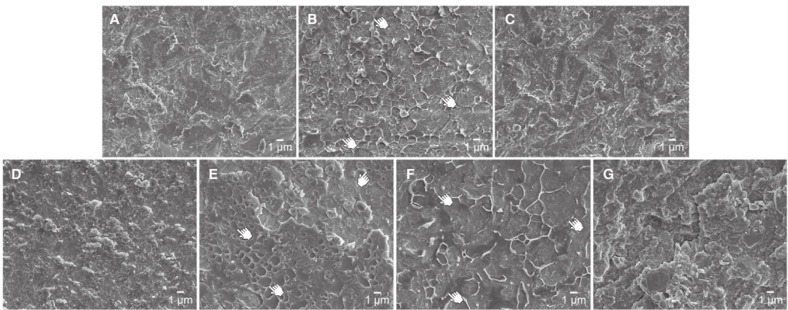
Fig. 4 shows representative SEM images of zirconia surfaces after debonding. Groups NC, AA, IC, and SHC show the typical air-abraded zirconia surfaces. For groups WS, SDS, and HP, blister-like bubble formations on the airabraded surfaces were observed.
Fig. 4. Representative SEM images of zirconia surfaces after debonding (original magnification: 4000×). A: NC (control); B: WS (water-spray); C: AA (air-abrasion); D: IC (Ivoclean); E: SDS (sodium dodecyl sulfate); F: HP (H2O2); and G: SHC (NaOCl). For groups WS, SDS, and HP (images B, E, and F), some blister-like bubble formations are indicated by pointers.
DISCUSSION
According to the manufacturer, IC contains sodium hydroxide and is meant for extraoral use only. The three substances used to prepare the cleaning solutions also have potentially adverse intraoral effects when present in high concentrations and with prolonged exposure. Clinically relevant concentrations of SDS are 0.015-1.5%, and toothpastes usually contain 1-3% of SDS as the detergent.19 Home mouth rinses and dentifrices contain low concentrations (1% or less) of HP.20 Although 5.25% SHC is a common tissue solvent, 1% SHC solution has effective tissuedissolving capability.21 Considering the potential use of such cleaning solutions for intraoral repair procedures with restorative composite resin, three experimental zirconiacleaning solutions with relatively low concentration (1.0 wt%) were prepared and tested (Fig. 1).
Non-covalent adsorption of salivary proteins, simulated by saliva immersion in this study, occurs on zirconia surface after try-in.1 Only water-spray rinsing of the specimens after saliva contamination significantly lowered the bond strength value, compared to the control (Table 3). The additional air-abrasion after contamination effectively removed saliva contamination without significantly increasing the Ra value (Table 2) and restored the bond strength significantly to the same level as that in the control, in accordance with some previous studies.1,4,5,6 In this study, moreover, some of the cleaning solutions (IC or SHC) were found to be also effective in removing saliva contamination and in enhancing the resin bond strengths. Thus, the null hypothesis that the cleaning solutions are less effective than air-abrasion in terms of resin bond strength was rejected.
Surface hydrophobicity/hydrophilicity can be determined by CA measurement.22 It is known that surface roughness also alters the CA values of the surface.9 In this study, the influence of surface roughness on the CA values can be excluded because the Ra values among the seven test groups were not significantly different (Table 1).9,10 Although zirconia is rather hydrophobic and has a low surface free energy,23 air-abrasion creates high surface energy and promotes microretention.24 Thus, air-abraded zirconia surfaces without saliva contamination may be considered relatively hydrophilic. According to the CA measurements (Fig. 2), groups NC, AA, IC, and SHC, which produced higher bond strength values (Table 3 and Table 4), showed lower advancing CA values (39.7-52.2°) and therefore indicated more hydrophilic surfaces. On the contrary, groups WS, SDS, and HP, which exhibited lower bond strength values, yielded lower CA values (62.9-65.2°) and indicated more hydrophobic surfaces. Thus, more hydrophilic zirconia surfaces indicate more effective cleaning; whereas more hydrophobic surfaces indicate less-effective cleaning.8 In addition, since chemical heterogeneity can also cause CA hysteresis,9 greater surface inhomogeneity due to less-effective cleaning of saliva-contaminated zirconia surfaces may induce greater CA hysteresis.8 Thus, a significantly strong negative correlation between the cosΘa and the CA hysteresis (Fig. 2) indicates that CA hysteresis is also relevant in assessing the cleanness of rough surfaces.8 Nonetheless, CA measurements alone are not sufficient to characterize surface chemical changes on zirconia before and after cleaning.22 Therefore, XPS was also used to identify the chemical elements on the surfaces. The depth and spatial resolutions for XPS are 1-25 nm and 8-150 µm, respectively.25 Since the subtended zirconia surface signal was detectable (Fig. 3), the thickness of the contamination layer was less than 10 nm.7
In dental practice, SHC solution has been widely used as an endodontic irrigant due to its effective antimicrobial and tissue-dissolving capabilities.26 It is also known that residual SHC may interfere with resin polymerization due to oxygen generation.27 Among the experimental cleaning solutions, however, 1% SHC solution was the most effective in removing the saliva contaminants from the zirconia surface (Table 3). XPS analysis also showed a lower O/Zr ratio for the zirconia surface cleaned with SHC than that cleaned with HP. SEM observation of the debonded surface revealed little bubble formation at the interface (Fig. 4G). These findings may indicate that SHC effectively cleaned the surface; water-spray rinsing then removed most of the residual SHC on the zirconia surface.
The results for group AA confirms again that air-abrasion is a useful cleaning method of saliva-contaminated zirconia.1,4,5,6 In clinical practice, however, the more complex surface geometry of zirconia-based restorations may make it difficult to remove contamination using air-abrasion.1 In such cases, a microbrush would be more convenient for applying cleaning solutions such as SHC to the inner surfaces of the restorations.
According to the manufacturer (Ivoclar Vivadent Scientific Documentation, 2011), the alkaline suspension of zirconium oxide particles in IC removes salivary phosphate contaminants by adsorption. Although 0.5% P element was detected in group WS, no P element was detected in group IC (Fig. 3). It appears that the application of IC effectively removed various contaminants (including salivary phosphate) from the surface and provided a clean surface for improved resin bonding (Table 3).
SDS is an anionic surfactant commonly used in the removal of proteins.28 However, group SDS showed a significantly lower bond strength than groups NC and AA (Tables 3 and 4). As stated above, groups WS, SDS, and HP showed similar higher water CAs (i.e., rather hydrophobic surfaces) (Fig. 2).29 Nonetheless, the CA hysteresis of group SDS was greater than that of group HP and similar to that of group WS. Similarly, XPS analysis showed a higher C/Zr ratio in group SDS than in group HP (5.6 vs. 3.5). These findings indicate less complete removal of carbon by 1% SDS solution when compared with 1% HP. Moreover, small bubbles remained on the SDS-treated and water-washed zirconia surface (Fig. 4E). Thus, the solution seems ineffective in cleaning the saliva-contaminated zirconia surface.
For group WS, the N element was detected on the zirconia surface (Table 2). After cleaning with the solutions, the N/Zr ratio was reduced but N remained on the zirconia surfaces. Nitrogen was not detected only in groups NC and AA, in which the surfaces were air-abraded. Groups IC and SHC showed lower cosΘa values and higher CA hystereses than group AA (Fig. 2). These findings might imply the superior cleaning potential of saliva-contaminated zirconia surface by air-abrasion than by the application of IC or 1% SHC, although no significant differences in shear bond strength were found among the three cleaning methods (Table 3). Further research is still needed to clarify whether the cleaning solutions are superior to air-abrasion in terms of long-term clinical bond strength.
HP is one of the principal reactive products of oxygen metabolism and often used as a bleach or cleaning agent. Although CA measurements and XPS analysis indicated a relatively effective cleaning efficacy of 1% HP, group HP exhibited the lowest bond strength value among the seven test groups. This may be due to the more extensive bubble formations on the zirconia surface after cleaning with HP (Fig. 4F).
In microtensile bond strength testing, each tooth can be considered a statistical unit because the variation within the tooth may be larger than the inter-tooth variation.30.31 In this study, a zirconia disk, which is expected to show less heterogeneity as opposed to tooth material, was used,31 three resin cylinders being bonded on one zirconia surface.22 In post hoc power analysis, a power of about 0.80 is regarded as acceptable for most purposes.32 For the bond strength data in this study, the power values were 1.00. In our linear mixed model, responses from a subject are thought to be the sum (linear) of fixed and random effects, the latter contributing only to the covariance structure of the data. Although the fixed effect was the primary interest in our study, it was necessary to adjust for the covariance. Thus, the statistical analyses for the shear bond strength data were done in two ways after transformation: under the assumption that the data are independent (ST 1) and that the data on one zirconia are correlated (ST 2).17 When the preset threshold value of alpha was set to 0.05, the statistical results in the pairwise comparisons were not affected by the methods of statistical analysis (i.e., STs 1 or 2). Nevertheless, the P values in some of the pairwise comparisons differed between the two methods. In particular, the bond strength of group SDS was "highly" significantly lower than those of groups AA and IC according to ST 1. The value of group HP was "marginally" significantly lower than that of group WS according to ST 1, but not significantly different from each other, according to ST 2. Although a zirconia ceramic is expected to show less heterogeneity as opposed to tooth material, it may thus be necessary to adjust for the covariance structure of the data.
During the try-in of zirconia restorations, the surfaces to be bonded may be additionally contaminated by blood or silicone indicators, which might also compromise resin bonding.1,4,6 These contaminants were not included in the present study and further experiments are needed to determine whether the cleaning solutions tested are also effective for such contamination. The cleaning efficacy of 1% SHC solution on other prosthetic restoration surfaces should also be studied. In addition, the safety of SHC should be assessed for intraoral use.
CONCLUSION
The findings of this study confirm that saliva contamination significantly reduces resin shear bond strength to zirconia and that air-abrasion is a useful cleaning method. However, a simple application of IC or 1% SHC effectively removed the saliva contaminants and provided a clean surface. The resin bond strength results were supported by water CA measurements and chemical identification of the zirconia surface with XPS. However, long-term clinical studies are still required to clarify the efficacy of the cleaning solutions in improving resin bonding of saliva-contaminated zirconia.
Footnotes
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2061732).
References
- 1.Yang B, Scharnberg M, Wolfart S, Quaas AC, Ludwig K, Adelung R, Kern M. Influence of contamination on bonding to zirconia ceramic. J Biomed Mater Res B Appl Biomater. 2007;81:283–290. doi: 10.1002/jbm.b.30664. [DOI] [PubMed] [Google Scholar]
- 2.Kim MJ, Kim YK, Kim KH, Kwon TY. Shear bond strengths of various luting cements to zirconia ceramic: surface chemical aspects. J Dent. 2011;39:795–803. doi: 10.1016/j.jdent.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Wegner SM, Kern M. Long-term resin bond strength to zirconia ceramic. J Adhes Dent. 2000;2:139–147. [PubMed] [Google Scholar]
- 4.Quaas AC, Yang B, Kern M. Panavia F 2.0 bonding to contaminated zirconia ceramic after different cleaning procedures. Dent Mater. 2007;23:506–512. doi: 10.1016/j.dental.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Yang B, Lange-Jansen HC, Scharnberg M, Wolfart S, Ludwig K, Adelung R, Kern M. Influence of saliva contamination on zirconia ceramic bonding. Dent Mater. 2008;24:508–513. doi: 10.1016/j.dental.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Yang B, Wolfart S, Scharnberg M, Ludwig K, Adelung R, Kern M. Influence of contamination on zirconia ceramic bonding. J Dent Res. 2007;86:749–753. doi: 10.1177/154405910708600812. [DOI] [PubMed] [Google Scholar]
- 7.Phark JH, Duarte S, Jr, Kahn H, Blatz MB, Sadan A. Influence of contamination and cleaning on bond strength to modified zirconia. Dent Mater. 2009;25:1541–1550. doi: 10.1016/j.dental.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Boulange-Petermann L, Joud JC, Baroux B. Wettability parameters controlling the surface cleanability of stainless steel. In: Mittal KL, editor. Contact angle, wettability and adhesion. Leiden: VSP; 2008. pp. 139–151. [Google Scholar]
- 9.Kim YK, Son JS, Kim KH, Kwon TY. Influence of surface energy parameters of dental self-adhesive resin cements on bond strength to dentin. J Adhesion Sci Technol. 2013;27:1778–1789. [Google Scholar]
- 10.Kim YK, Min BK, Son JS, Kim KH, Kwon TY. Influence of different drying methods on microtensile bond strength of self-adhesive resin cements to dentin. Acta Odontol Scand. 2014;72:954–962. doi: 10.3109/00016357.2014.926024. [DOI] [PubMed] [Google Scholar]
- 11.Takimoto M, Ishii R, Iino M, Shimizu Y, Tsujimoto A, Takamizawa T, Ando S, Miyazaki M. Influence of temporary cement contamination on the surface free energy and dentine bond strength of self-adhesive cements. J Dent. 2012;40:131–138. doi: 10.1016/j.jdent.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Chibowski E, Hołysz L, Terpilowski K, Jurak M. Investigation of super-hydrophobic effect of PMMA layers with different fillers deposited on glass support. Colloids Surf A: Physicochem Eng Asp. 2006;291:181–190. [Google Scholar]
- 13.Hołysz L, Mirosław M, Terpiłowski K, Szcześ A. Influence of relative humidity on the wettability of silicon wafer surfaces. Ann UMCS Chem. 2008;63:223–239. [Google Scholar]
- 14.Phark JH, Duarte S, Jr, Blatz M, Sadan A. An in vitro evaluation of the long-term resin bond to a new densely sintered high-purity zirconium-oxide ceramic surface. J Prosthet Dent. 2009;101:29–38. doi: 10.1016/S0022-3913(08)60286-3. [DOI] [PubMed] [Google Scholar]
- 15.Hagge MS, Lindemuth JS. Shear bond strength of an autopolymerizing core buildup composite bonded to dentin with 9 dentin adhesive systems. J Prosthet Dent. 2001;86:620–623. doi: 10.1067/mpr.2001.119683. [DOI] [PubMed] [Google Scholar]
- 16.Yun JY, Ha SR, Lee JB, Kim SH. Effect of sandblasting and various metal primers on the shear bond strength of resin cement to Y-TZP ceramic. Dent Mater. 2010;26:650–658. doi: 10.1016/j.dental.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Sattabanasuk V, Vachiramon V, Qian F, Armstrong SR. Resin-dentin bond strength as related to different surface preparation methods. J Dent. 2007;35:467–475. doi: 10.1016/j.jdent.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 18.McClean MD, Rinehart RD, Ngo L, Eisen EA, Kelsey KT, Wiencke JK, Herrick RF. Urinary 1-hydroxypyrene and polycyclic aromatic hydrocarbon exposure among asphalt paving workers. Ann Occup Hyg. 2004;48:565–578. doi: 10.1093/annhyg/meh044. [DOI] [PubMed] [Google Scholar]
- 19.Neppelberg E, Costea DE, Vintermyr OK, Johannessen AC. Dual effects of sodium lauryl sulphate on human oral epithelial structure. Exp Dermatol. 2007;16:574–579. doi: 10.1111/j.1600-0625.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 20.Walsh LJ. Safety issues relating to the use of hydrogen peroxide in dentistry. Aust Dent J. 2000;45:257–269. doi: 10.1111/j.1834-7819.2000.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod. 1992;18:605–612. doi: 10.1016/S0099-2399(06)81331-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Suh BI, Brown D, Chen X. Bonding of primed zirconia ceramics: evidence of chemical bonding and improved bond strengths. Am J Dent. 2012;25:103–108. [PubMed] [Google Scholar]
- 23.Piascik JR, Swift EJ, Braswell K, Stoner BR. Surface fluorination of zirconia: adhesive bond strength comparison to commercial primers. Dent Mater. 2012;28:604–608. doi: 10.1016/j.dental.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Matinlinna JP, Heikkinen T, Ozcan M, Lassila LV, Vallittu PK. Evaluation of resin adhesion to zirconia ceramic using some organosilanes. Dent Mater. 2006;22:824–831. doi: 10.1016/j.dental.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 25.Ratner BD. Characterization of biomaterial surfaces. Cardiovasc Pathol. 1993;2:87–100. [Google Scholar]
- 26.Spencer HR, Ike V, Brennan PA. Review: the use of sodium hypochlorite in endodontics-potential complications and their management. Br Dent J. 2007;202:555–559. doi: 10.1038/bdj.2007.374. [DOI] [PubMed] [Google Scholar]
- 27.Del Carpio-Perochena AE, Bramante CM, Duarte MA, Cavenago BC, Villas-Boas MH, Graeff MS, Bernardineli N, de Andrade FB, Ordinola-Zapata R. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J Endod. 2011;37:1134–1138. doi: 10.1016/j.joen.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Liu G, Craig VS. Improved cleaning of hydrophilic proteincoated surfaces using the combination of Nanobubbles and SDS. ACS Appl Mater Interfaces. 2009;1:481–487. doi: 10.1021/am800150p. [DOI] [PubMed] [Google Scholar]
- 29.Arkles B, Pan Y, Kim YM. The role of polarity in the structure of silanes employed in surface modification. In: Mittal KL, editor. Silanes and other coupling agents. Leiden: CRC Press; 2009. pp. 51–64. [Google Scholar]
- 30.Vanderlei A, Passos SP, Özcan M, Bottino MA, Valandro LF. Durability of adhesion between feldspathic ceramic and resin cements: effect of adhesive resin, polymerization mode of resin cement, and aging. J Prosthodont. 2013;22:196–202. doi: 10.1111/j.1532-849X.2012.00934.x. [DOI] [PubMed] [Google Scholar]
- 31.Roulet JF. Statistics: nuisance - tool - necessity. J Adhes Dent. 2013;15:203. doi: 10.3290/j.jad.a29842. [DOI] [PubMed] [Google Scholar]
- 32.De Lucas M, Janss GFE, Whitfield DP, Ferrer M. Collision fatality of raptors in wind farms does not depend on raptor abundance. J Appl Ecol. 2008;45:1695–1703. [Google Scholar]