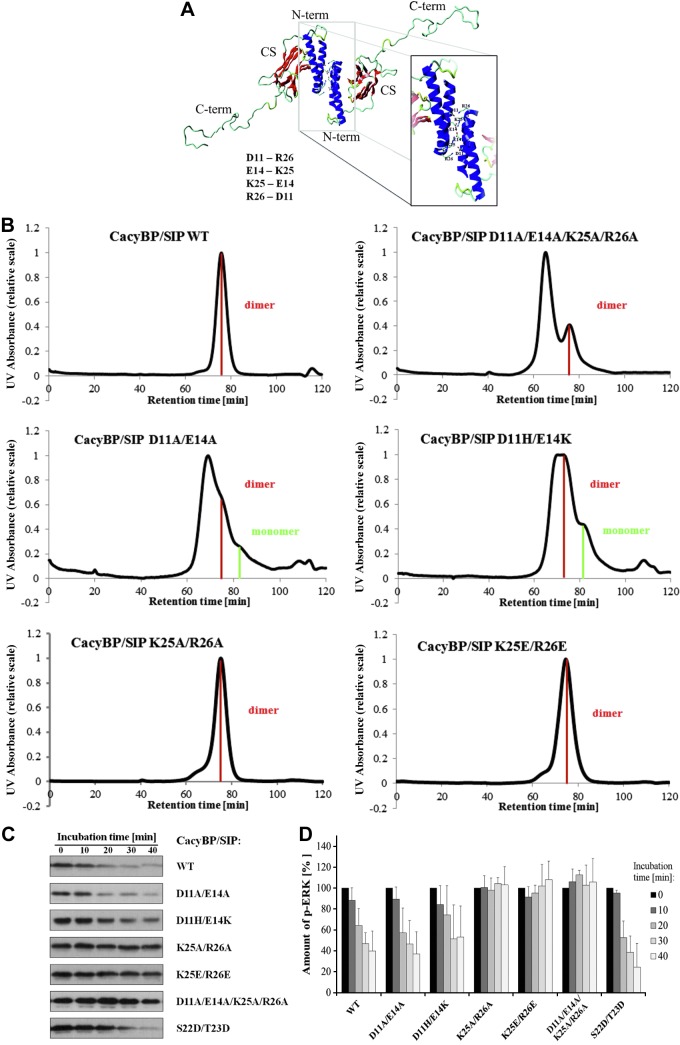
Figure 5.
Analysis of CacyBP/SIP dimer formation. A) Three-dimensional homology model of full-length CacyBP/SIP with the putative residues involved in the dimerization. N-term, N-terminal domain; CS, CS domain; C-term, C-terminal domain. B) Comparison of SEC chromatograms for CacyBP/SIP mutants within the N-terminal domain. Peak corresponding to the protein dimer is marked in red, protein monomer in green. C) Effect of mutations within the N-terminal domain of full-length CacyBP/SIP on its phosphatase activity and ERK1/2 binding. The activity assay for full-length CacyBP/SIP and its mutants analyzed by Western blot developed using anti-p-ERK1/2 antibody. D) Densitometric analysis for the phosphatase activity assay of full-length CacyBP/SIP and its mutants within the N-terminal domain. Statistical analysis was performed for 5 independent experiments.