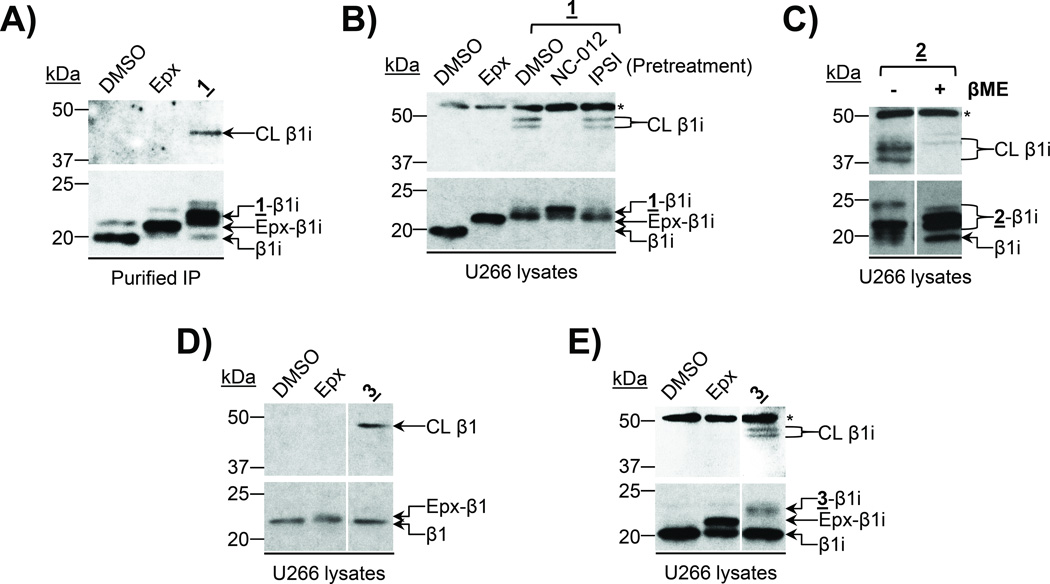
Figure 3.
UKP13-C6-LKS01 (probe 1) and UKP13-(PEG)4-NC012 (probe 3) crosslink β1/β1i with β2/β2i. A) The purified 20S immunoproteasome (IP) was treated with DMSO, epoxomicin (Epx), or probe 1 prior to immunoblotting for β1i. B) U266 cell lysates were treated with DMSO or epoxomicin, or pretreated with DMSO or the indicated subunit-selective inhibitors prior to treatment with probe 1 as indicated. β1i was detected by immunoblotting. C) High-molecular-weight immunoblot signals detected following treatment with bifunctional proteasome probes result from crosslinking of proteasome catalytic subunits. U266 cell lysates were treated with probe 2and proteins were subsequently denatured in sample buffer with or without β-mercaptoethanol (βME), which cleaves the internal disulfide bond of the linker. β1i was detected by immunoblotting. D) U266 cell lysates were incubated with DMSO, epoxomicin, or probe 3 prior to immunoblotting for β1. E) U266 cell lysates were incubated with DMSO, epoxomicin, or probe 3 prior to immunoblotting for β1i. Irrelevant lanes were cropped out of blots shown in C–E. CL = crosslinked β-subunit, * = non-specific band.