Abstract
Background
An epidemiological rise of allergic diseases in developing countries raises new challenges. Currently a paucity of data exists describing allergy symptomology and sensitization to common food and aeroallergens in young children from developing countries.
Objective
To compare changes in symptomology, food allergen sensitization and aeroallergen sensitization in a cross-sectional study of children <2 years and 2-10 years.
Methods
A total of 192 allergic children (aged <2 years, 35 children; aged 2-10 years, 157 children) underwent specific IgE (>0.35 kU/L) to common food (egg white, cow's milk, cod fish, wheat, peanut, soya, peanut, and shrimp) and house dust mites (Dermatophagoides pteronyssinus and Blomia tropicalis).
Results
In children <2 years, atopic dermatitis (65.7%) was the most common symptom whereas in children 2-10 years it was rhinoconjunctivitis (74.5%). Higher sensitization rate to eggs (p < 0.01) and cow's milk (p = 0.044) was seen in <2 years group when compared to the 2-10 years group, but no significant differences for shrimp (p = 0.29), wheat (p = 0.23) and soya (p = 0.057). Interestingly, sensitization to peanut (p = 0.012) and fish (p = 0.035) was significantly decreased in the 2-10 years group. Sensitization to house dust mites (p < 0.01) dramatically increased in the older children.
Conclusion
Our study supports concept of atopic march from a developing country like Malaysia.
Keywords: Hypersensitivity, Allergens, Asia, Food, Pyroglyphidae
INTRODUCTION
Recent decades have witnessed pronounced increasing prevalence of allergic disease in South East Asia [1]. Atopic march describes a sequential progression of atopic dermatitis and food allergy in infants leading to chronic respiratory conditions such as allergic rhinitis and asthma in school aged children. Risk of developing atopic diseases is complex, hence temporal patterns described in the atopic march is typically not a simple progression but influenced by genetic and environmental factors.
Prevalence of food allergy is increasing in developed nations but data from many parts of developing Asia are scant, creating a perception of low prevalence though recent reports indicate otherwise [2]. Asia's diverse cultures and unique eating habits further raise the question if current understanding of food allergy, largely derived from Western populations, are relevant to the region. Indeed, food sensitization with food-specific IgE level proposed predictive values for clinical food allergy, was not replicated in Chinese children [3]. Reactions to egg, peanut, milk, tree nuts, soy, fish, and wheat are common in Asians [3], however epidemiological studies from the region till date have primarily been survey based, lacking objective measures, and focussed on older children when sensitization to certain foods could be outgrown.
In a prospective birth cohort study, food allergen sensitization decreased from 10% to 3%, whilst inhalant allergen sensitization increased from 1.5% to 8%, by 6 years [4]. Despite paucity of similar prospective studies from Asia, co-occurrence of food allergy may accelerate the atopic march in Japanese children [5]. Japanese infants sensitized to house dust mites and co-sensitized to food antigens were at greatest risk for asthma development in a 4 year follow-up period [6].
Most studies to date from Asia were from affluent countries sharing comparable socioeconomic indices as Western Europe which may not accurately reflect the populous developing region. Indeed, geographical location emerged as an independent risk factor for development of allergies and asthma spanning three South East Asian nations [7], hence a need for data from regional emerging economies [8]. This cross-sectional study of sensitization and symptomology patterns of Malaysian allergic children was undertaken to draw parallels with the atopic march and how it applies to our population. We attempt to provide an insight on how sensitization to common food and aero-allergens may evolve in children of a developing Asian country such as Malaysia in comparison to other populations from more developed nations.
MATERIALS AND METHODS
After approval from the ethical review board, 192 allergic children (aged < 2 years, 35 children; aged 2 to 10 years, 157 children) evaluated at an allergy clinic in Pantai Hospital, Kuala Lumpur were enrolled in this retrospective study. All children with allergic symptoms over a three years span were included. The parents were interviewed based on a standard questionnaire that included self reported clinical history of allergy symptoms (gastroenterological, respiratory, dermatological, otorhinolaryngological, and ophthalmological), family atopy history, home environmental factors and diet of the child. When multiple symptoms were present in the individual, each symptom was considered separately.
Based on clinical assessment by an allergy specialist, sensitization was then confirmed by positive specific IgE (>0.35 kU/L) to common food allergens such as egg white, cow's milk, cod fish, wheat, peanut, soya bean, peanut or shrimp, and house dust mite (Dermatophagoides pteronyssinus and Blomia tropicalis) by ImmunoCAP (Pharmacia, Uppsala, Sweden). Pollens were not evaluated in this study as they are not a major aero-allergen source in Malaysia.
The chi-square test was used to compare children <2 years and 2-10 years in relation to symptoms, food and dust mite sensitization. A p value <0.05 was considered statistically significant (SPSS ver. 11.0, SPSS Inc., Chicago, IL, USA).
RESULTS
Table 1 summarizes prominent symptoms in the group of children tested for allergy. Except for atopic dermatitis and gastrointestinal complaints, all other symptoms were evidently higher in children aged 2 to 10 years when compared to infants lesser than 2 years old. The frequencies of upper respiratory symptoms such as allergic rhinitis (74.5% vs. 34.3%) and lower respiratory symptoms such as asthma (36.9% vs. 22.9%) were significantly higher in older children compared to the younger group. Gastrointestinal symptom was more commonly seen in children less than 2 years (20%) against children (13.4%) 2-10 years. The frequency of atopic dermatitis was higher in children less than 2 years (65.7%) compared to children 2-10 years (58%); though these were statistically not significant.
Table 1. Frequency of clinical symptoms in children aged 2 to 10 years compared to infants less than 2 years old.
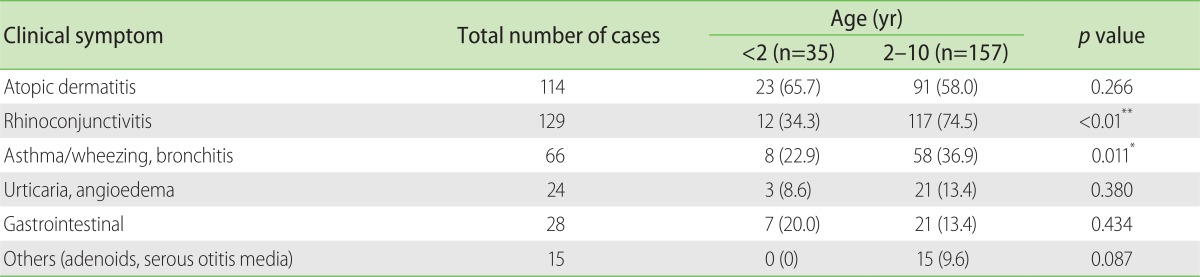
Values are presented as number (%).
*p < 0.05. **p < 0.01. Chi-square test.
Of those tested for food allergens (Table 2), younger children <2 years were significantly more likely to be sensitized to egg white (p < 0.01), cow's milk (p = 0.044), cod fish (p = 0.035) and peanut (p = 0.012), but not to soya (p = 0.057), wheat (p = 0.23) or shrimp (p = 0.29). Children in the <2 years group had higher sensitization rates for all tested food compared to older children including egg white (60% vs. 26.2%), cow's milk (44.4% vs. 25.7%), cod fish (23.8% vs. 6.6%), wheat (23.1% vs.11.8%), peanut (36.4% vs. 12.6%) and soya (25% vs. 9.8%) except for shrimp (7.7% vs. 25.2%). Older children had significantly higher sensitization rates (p < 0.01) to D. pteronyssinus (63.3% vs. 23.1%) and B. Tropicalis (63.2% vs. 16.7%) compared to younger children (Table 3).
Table 2. Food allergen sensitization in allergic children aged 2 to 10 years compared to infants less than 2 years old.
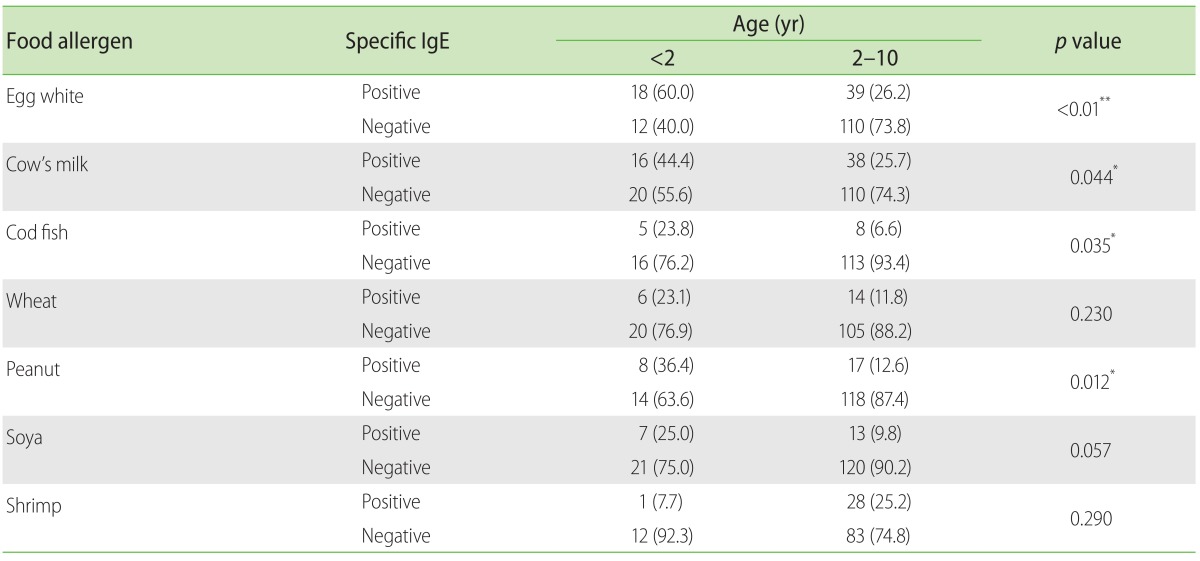
Values are presented as number (%).
*p < 0.05. **p < 0.01. Chi-square test
Table 3. Dust mite sensitization in allergic children aged 2 to 10 years compared to infants less than 2 years old.
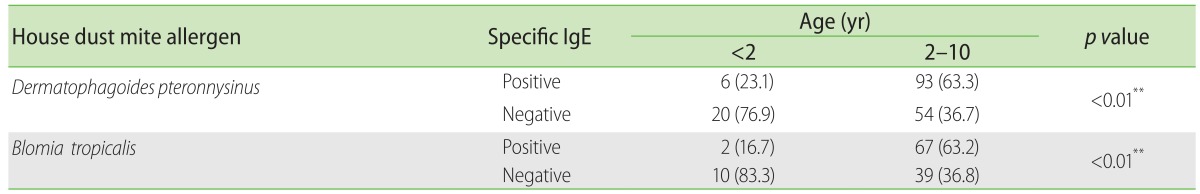
Values are presented as number (%).
**p < 0.01. Chi-square test.
DISCUSSION
Epidemiological studies till date on prevalence of allergic disease from Asia have focused on older children with dearth of data in infants, stratification of symptoms and sensitization trends to common food and aero-allergens.
Atopic dermatitis is generally the first clinical manifestation of the atopic march. The most frequent allergy symptoms include atopic dermatitis in children below 2 years of age (65.7%) and rhinoconjunctivitis (74.5%) in children 2-10 years. Allergy symptomology in Singaporean children during second year of life primarily featured chronic itchy rash, wheeze and rhinoconjunctivitis [9], however allergic rhinitis developed after 6 years of age and rarely <2 years of age [10], in concordance to our observation. Kapoor et al. [11] examined the prevalence of allergic rhinitis and asthma in 2,270 children with physician-confirmed atopic dermatitis, and by 3 years of age 66% manifested allergic rhinitis and/or asthma. Asthma in early childhood is difficult to differentiate from other notable causes of symptoms such as viral infections, nonetheless despite an overestimation of asthma symptoms in children <2 years group, significant increase in respiratory manifestation among older children was present. Prognosis of atopic dermatitis may vary due to genetic and environmental influence. Atopic dermatitis incidence was higher in the younger age group but not significant between both age groups. There are no longitudinal studies from Asia to describe the natural course of atopic dermatitis, though 70% were in remission by 5 years of age in a retrospective review of Korean infants; albeit late onset atopic dermatitis unaccounted for [12].
Atopic children are more likely to develop food allergy than nonatopic; upto 37% with atopic dermatitis having IgE-mediated food allergy [13]. Cow's milk and egg were the most common food allergens in Malaysian children, as also noted for the region [14]. Food allergy, particularly cow's milk and egg white protein allergy begins early in life but typically outgrown by 2 to 3 years, whereas clinical sensitivity to peanut persists life-long [15]. Sensitization to all tested food allergens, except shrimp, demonstrated a decreasing trend in older children. As a consequence, cow's milk and egg were less likely to be positive in older children in relation to younger children, suggestive of tolerance development.
Previously recognized, wheat and soya allergy being outgrown by preschool-age, on more recent studies focusing on natural course indicate slower progression towards desensitization by late childhood or adolescence, often trailing clinical oral tolerance [16,17,18,19]. These support our findings of decreasing sensitization in older children but not achieving significance, possibly indicative of a protracted desensitization course.
Data on fish allergy in Asia remains scant. From a pool of 7,850 Singaporean children with suspected food allergy only 10 had fish allergy, raising the notion that despite high consumption, fish allergy in the region may be uncommon [20]. Major allergen parvalbumin in locally consumed tropical fish is highly cross-reactive to cod fish, rendering cod fish IgE commercial testing reliable [20]. The natural course of fish allergy is poorly understood though prevailing consensus is it remains persistent. Surprisingly, we observe a significant drop in fish sensitization among older children, seemingly a propensity to outgrow sensitization in our study population. Regular fish consumption within the first year of life, but not later, attenuates atopic tendencies by age 4 years [21,22]. Breast feeding practices, which finds higher acceptance in developing nations, is further a confounding factor to the beneficial immune modulatory properties of early fish consumption [23]. Fish establishes early into diets of allergic Asian children [2], contrasting to Western practices [23]. Fish allergy was more prevalent in the Philippines (2.29%) compared to Singapore (0.26%) and Thailand (0.29%) on a survey based study in adolescents [24]. Hence role of food processing, dietary habits and cultural practices in fish allergy warrants investigation.
Population based peanut allergy prevalence are generally lower in Asians than Western societies [25]. A higher self-reported peanut but lower shrimp allergy amongst Western born expatriate in comparison to local Asians highlight the stark differences [26]. Nonetheless, peanut sensitized Asian children possess similar clinical and immunological traits as their Western counterparts [27]. We find a sharp drop in peanut sensitization in older children, contrasting with a significant rise in shrimp sensitization. Similar trends have been reported in Singaporean children [2]. This may suggest Malaysian children have a tendency of outgrowing peanut sensitization, lending support towards mounting evidence that peanut allergy in Asia maybe distinct from the West. It is tempting to implicate genetic, dietary habits and environmental factors for the disparities.
Shellfish allergy has traditionally been perceived as persistent, as suggested also by this study. There are two potential pathways for sensitization to shellfish. First, early introduction of the highly allergenic shrimp paste condiment widely used in the local diet. Secondly, the tropical Malaysian climate promotes abundant exposure to dust mite allergens within the indoor environment. In Malaysian allergic rhinitis children, house dust mite and shrimp are the most common aero- and food-allergens, respectively [28].
Hence cross-reacting tropomyosin of dust mite allergens may impart shellfish sensitization in our population. B. tropicalis and D. pteronyssinus are the predominant reported house dust mites in Malaysian homes [29]. Prospective studies from Asia demonstrate persistently elevated food specific-IgE and early childhood house dust mite sensitization as risk factors for subsequent asthma development [6]. House dust mite sensitization is associated with asthma and allergic rhinitis in Malaysian school aged children [7]. We find dramatic sensitization increases to D. pteronyssinus and B. Tropicalis in older children contributing to respiratory symptoms.
Some limitations need to be taken into account including a single institute experience and a cross-sectional study. Allergen testing were based on clinical assessment and response by parents, hence not all children were tested to the allergen panel. While longitudinal studies would eventually need to be carried out, the current study defines commonly associated allergenic sources and offers inimitable insight of childhood sensitization trends from a developing country within the region.
In summary a gradual transition from atopic dermatitis to predominantly respiratory symptoms, a decreasing trend of sensitization to common food allergens contrasting with rising aero-allergen sensitization associated with progression of age, support the atopic march concept in allergic Malaysian children. Development of tolerance to peanut sensitization and an increasing sensitization to shrimp maybe unique features to the region requiring further investigation.
References
- 1.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR; ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–954.e15. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Chiang WC, Kidon MI, Liew WK, Goh A, Tang JP, Chay OM. The changing face of food hypersensitivity in an Asian community. Clin Exp Allergy. 2007;37:1055–1061. doi: 10.1111/j.1365-2222.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 3.Leung TF, Lam CW, Chan IH, Li AM, Tang NL. Sensitization to common food allergens is a risk factor for asthma in young Chinese children in Hong Kong. J Asthma. 2002;39:523–529. doi: 10.1081/jas-120004922. [DOI] [PubMed] [Google Scholar]
- 4.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–1179. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 5.Kijima A, Murota H, Takahashi A, Arase N, Yang L, Nishioka M, Yamaoka T, Kitaba S, Yamauchi-Takihara K, Katayama I. Prevalence and impact of past history of food allergy in atopic dermatitis. Allergol Int. 2013;62:105–112. doi: 10.2332/allergolint.12-OA-0468. [DOI] [PubMed] [Google Scholar]
- 6.Ohshima Y, Yamada A, Hiraoka M, Katamura K, Ito S, Hirao T, Akutagawa H, Kondo N, Morikawa A, Mayumi M. Early sensitization to house dust mite is a major risk factor for subsequent development of bronchial asthma in Japanese infants with atopic dermatitis: results of a 4-year followup study. Ann Allergy Asthma Immunol. 2002;89:265–270. doi: 10.1016/S1081-1206(10)61953-9. [DOI] [PubMed] [Google Scholar]
- 7.Leung R, Ho P, Lam CW, Lai CK. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol. 1997;99:594–599. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 8.Boye JI. Food allergies in developing and emerging economies: need for comprehensive data on prevalence rates. Clin Transl Allergy. 2012;2:25. doi: 10.1186/2045-7022-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan TN, Lim DL, Lee BW, Van Bever HP. Prevalence of allergy-related symptoms in Singaporean children in the second year of life. Pediatr Allergy Immunol. 2005;16:151–156. doi: 10.1111/j.1399-3038.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Chiang WC, Chen YM, Tan HK, Balakrishnan A, Liew WK, Lim HH, Goh SH, Loh WY, Wong P, Teoh OH, Goh A, Chay OM. Allergic rhinitis and non-allergic rhinitis in children in the tropics: prevalence and risk associations. Pediatr Pulmonol. 2012;47:1026–1033. doi: 10.1002/ppul.22554. [DOI] [PubMed] [Google Scholar]
- 11.Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68–73. doi: 10.1016/j.jaad.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 12.Chung Y, Kwon JH, Kim J, Han Y, Lee SI, Ahn K. Retrospective analysis of the natural history of atopic dermatitis occurring in the first year of life in Korean children. J Korean Med Sci. 2012;27:723–728. doi: 10.3346/jkms.2012.27.7.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.3.e8. [DOI] [PubMed] [Google Scholar]
- 14.Hill DJ, Hosking CS, Zhie CY, Leung R, Baratwidjaja K, Iikura Y, Iyngkaran N, Gonzalez-Andaya A, Wah LB, Hsieh KH. The frequency of food allergy in Australia and Asia. Environ Toxicol Pharmacol. 1997;4:101–110. doi: 10.1016/s1382-6689(97)10049-7. [DOI] [PubMed] [Google Scholar]
- 15.Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, Plaut M, Cooper SF, Fenton MJ, Arshad SH, Bahna SL, Beck LA, Byrd-Bredbenner C, Camargo CA, Jr, Eichenfield L, Furuta GT, Hanifin JM, Jones C, Kraft M, Levy BD, Lieberman P, Luccioli S, McCall KM, Schneider LC, Simon RA, Simons FE, Teach SJ, Yawn BP, Schwaninger JM NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol. 2010;126:1105–1118. doi: 10.1016/j.jaci.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA. The natural history of wheat allergy. Ann Allergy Asthma Immunol. 2009;102:410–415. doi: 10.1016/S1081-1206(10)60513-3. [DOI] [PubMed] [Google Scholar]
- 17.Mansouri M, Pourpak Z, Mozafari H, Abdollah Gorji F, Shokouhi Shoormasti R. Follow-up of the wheat allergy in children; consequences and outgrowing the allergy. Iran J Allergy Asthma Immunol. 2012;11:157–163. [PubMed] [Google Scholar]
- 18.Kotaniemi-Syrjanen A, Palosuo K, Jartti T, Kuitunen M, Pelkonen AS, Makela MJ. The prognosis of wheat hypersensitivity in children. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e421–e428. doi: 10.1111/j.1399-3038.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 19.Savage JH, Kaeding AJ, Matsui EC, Wood RA. The natural history of soy allergy. J Allergy Clin Immunol. 2010;125:683–686. doi: 10.1016/j.jaci.2009.12.994. [DOI] [PubMed] [Google Scholar]
- 20.Lim DL, Neo KH, Yi FC, Chua KY, Goh DL, Shek LP, Giam YC, Van Bever HP, Lee BW. Parvalbumin: the major tropical fish allergen. Pediatr Allergy Immunol. 2008;19:399–407. doi: 10.1111/j.1399-3038.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- 21.Nafstad P, Nystad W, Magnus P, Jaakkola JJ. Asthma and allergic rhinitis at 4 years of age in relation to fish consumption in infancy. J Asthma. 2003;40:343–348. doi: 10.1081/jas-120018633. [DOI] [PubMed] [Google Scholar]
- 22.Kiefte-de Jong JC, de Vries JH, Franco OH, Jaddoe VW, Hofman A, Raat H, de Jongste JC, Moll HA. Fish consumption in infancy and asthma-like symptoms at preschool age. Pediatrics. 2012;130:1060–1068. doi: 10.1542/peds.2012-0875. [DOI] [PubMed] [Google Scholar]
- 23.Kull I, Bergstrom A, Lilja G, Pershagen G, Wickman M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy. 2006;61:1009–1015. doi: 10.1111/j.1398-9995.2006.01115.x. [DOI] [PubMed] [Google Scholar]
- 24.Connett GJ, Gerez I, Cabrera-Morales EA, Yuenyongviwat A, Ngamphaiboon J, Chatchatee P, Sangsupawanich P, Soh SE, Yap GC, Shek LP, Lee BW. A population-based study of fish allergy in the Philippines, Singapore and Thailand. Int Arch Allergy Immunol. 2012;159:384–390. doi: 10.1159/000338940. [DOI] [PubMed] [Google Scholar]
- 25.Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac Allergy. 2013;3:3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shek LP, Cabrera-Morales EA, Soh SE, Gerez I, Ng PZ, Yi FC, Ma S, Lee BW. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126:324–331. 331.e1–331.e7. doi: 10.1016/j.jaci.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Chiang WC, Pons L, Kidon MI, Liew WK, Goh A, Wesley Burks A. Serological and clinical characteristics of children with peanut sensitization in an Asian community. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e429–e438. doi: 10.1111/j.1399-3038.2009.00930.x. [DOI] [PubMed] [Google Scholar]
- 28.Gendeh BS, Mujahid SH, Murad S, Rizal M. Atopic sensitization of children with rhinitis in Malaysia. Med J Malaysia. 2004;59:522–529. [PubMed] [Google Scholar]
- 29.Mariana A, Ho TM, Sofian-Azirun M, Wong AL. House dust mite fauna in the Klang Valley, Malaysia. Southeast Asian J Trop Med Public Health. 2000;31:712–721. [PubMed] [Google Scholar]


