Figure 1.
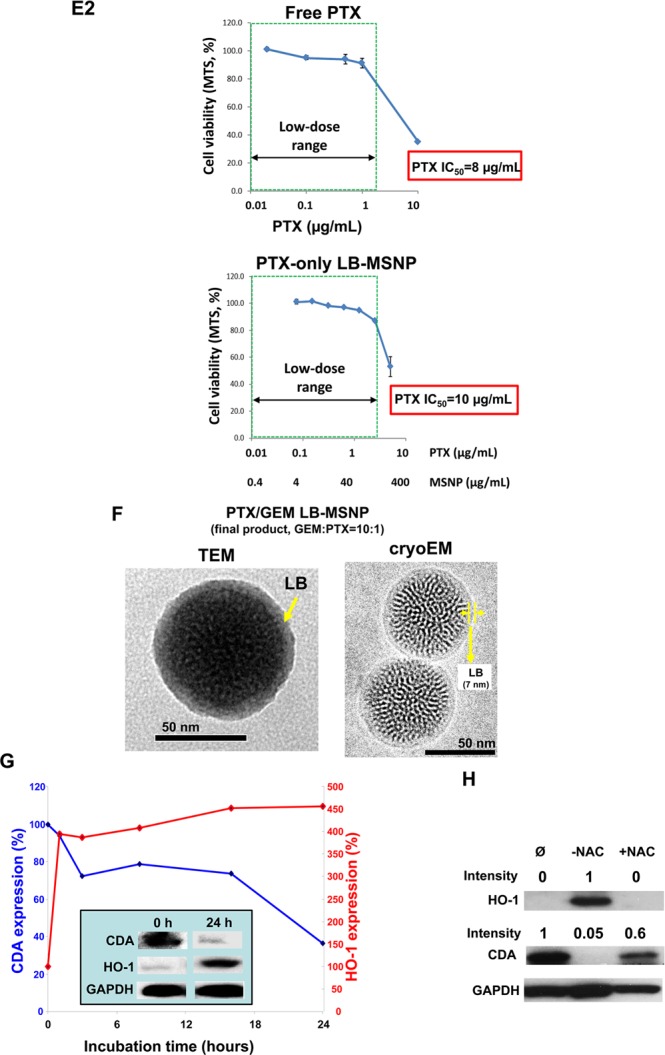
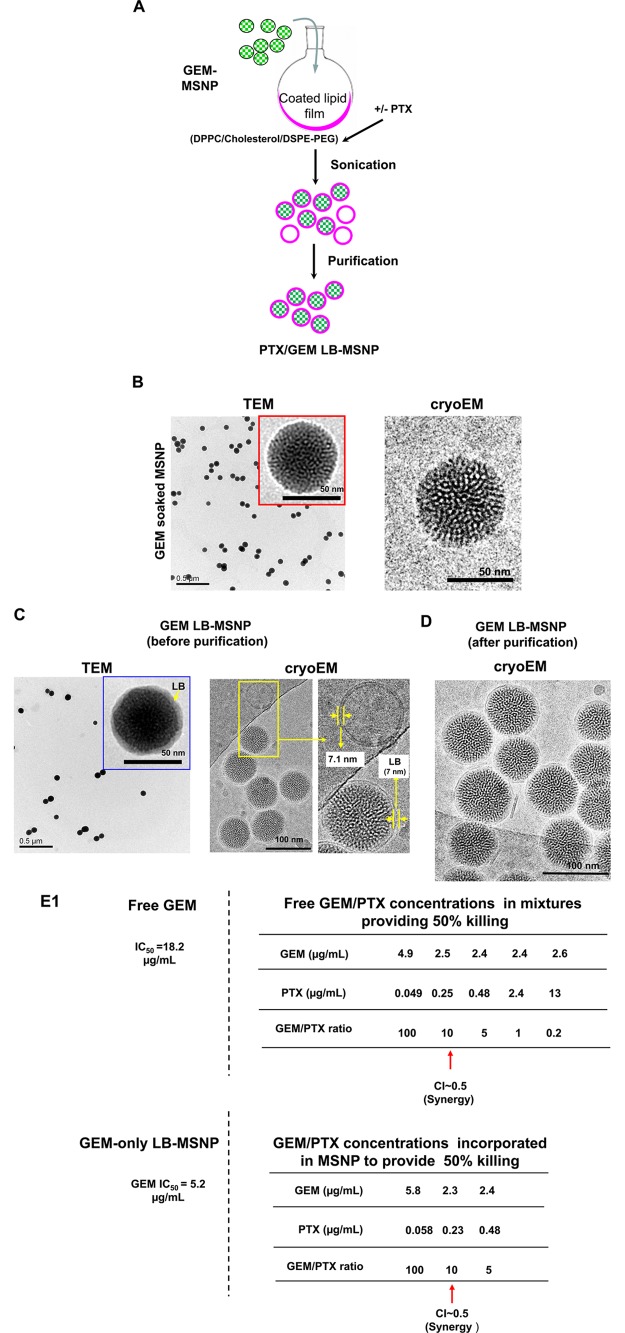
Synthesis, physicochemical characterization, and in vitro effects of PTX/GEM-loaded LB-MSNP. (A) Scheme depicting the procedure for LB-MSNP synthesis as a carrier for delivery of GEM only or a combination of GEM and PTX. MSNPs were synthesized by a sol/gel method. We further developed a coated lipid film procedure in which GEM-soaked MSNP suspensions were added to a continuous lipid film coated onto a glass surface, allowing uniform particle coating upon sonication. The coated lipid film was developed by mixing dipalmitoylphosphatidylcholine (DPPC)/cholesterol/1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)-PEG at a 77.5:20:2.5 molar ratio. After sonication, the particle suspension was purified by centrifugation and washed to separate the coated particles from liposomes and free drug. LB coating also allowed copackaging of the hydrophobic drug, PTX, with GEM trapped in the pores. This allowed ratiometric incorporation of 25 wt % GEM combined with 0–5 wt % PTX. (B) TEM (left) and cryoEM (right) images of GEM-soaked MSNPs before LB coating. The MSNP cores show ordered helical hexagonal pore arrangements. (C) TEM (left) and cryoEM (right) images of GEM-loaded LB-coated MSNPs before purification. The images demonstrate a particle size of 75 nm with uniform and intact LB, with 7 nm thickness; this is equivalent to the thickness of the lipid bilayer in liposomes (7.1 nm) that coform in the synthesis process. (D) CryoEM picture of GEM LB-MSNP after centrifugation, purification, and washing. (E) Ratiometric design of PTX/GEM co-delivery. (E1) Series of GEM/PTX combinations at different mixing ratios (100:1 to 0.2:1) were prepared as described in the Supporting Information S2. Each mixture was considered as a starting concentration to make a series of dilutions. Using the samples to conduct MTS experiments, we calculated the 50% killing concentrations of these mixtures as shown in the top panel in E1. To keep the dose of PTX in the low range, we decided on using a ratio of 10:1 in further experimentation. At this ratio, the free PTX concentration is 0.25 μg/mL, and that of GEM is 2.5 μg/mL (E, top panel). CompuSyn software was used to calculate combination index (CI). We showed a CI of 0.5 at a 10:1 ratio, which is indicative of strong synergy. The additional evaluation of the GEM/PTX synergy of the carrier is performed. A series of GEM/PTX LB-MSNPs were prepared using different encapsulation ratios (100:1, 10:1, and 5:1). Each particle type was used to assess cytotoxicity and determine the concentration of each drug in the mixture for a 50% killing effect (see E1, bottom panel). At an encapsulation ratio of 10:1, we reduced the GEM concentration required for 50% cell killing from 5.2 to 2.3 μg/mL. This occurred in the presence of a PTX dose of 0.23 μg/mL, which is nontoxic. (E2) Independent cell killing (MTS) experiments using free PTX or PTX-only LB-MSNP in PANC-1 cells. (F) TEM (left) and cryoEM (right) images of the ratiometric-designed PTX/GEM LB-MSNP, which at the ratio of 10:1 was used in cellular and animal studies. (G) Detection of CDA and heme oxygenase 1 (HO-1) expression by PTX/GEM LB-MSNP using Western blotting. PANC-1 cells were treated with PTX/GEM LB-MSNP (particle dose = 25 μg/mL; GEM = 6.25 μg/mL; PTX = 0.625 μg/mL) in complete DMEM medium for 0–24 h. The CDA and HO-1 expression was determined by immunoblotting. The relative density of the protein bands were determined by ImageJ software. Representative immunoblot bands at 0 and 24 h are shown in the inset. (H) Pretreatment of PANC-1 cells using N-acetylcysteine at 1.5 mg/mL interfered with the effects of dual drug delivery on CDA and HO-1 expression.