Table 2.
Parameter estimates of the final pharmacokinetic model
| Parameter | Fixed effects estimate | RSE (%)* | Random effects estimate† | RSE (%)‡ | Shrinkage (%) |
|---|---|---|---|---|---|
| CL (l h−1) | 1.21 × (WT/70)0.75 | 5.72 | %CV = 19.3 | 23.6 | 38 |
| Vc (l) | 59.3 × (WT/70)1.0 | 7.03 | %CV = 24.5 | 33.8 | 12 |
| Q (l) | 1.02 × (WT/70)0.75 | 29 | |||
| Vp (l) | 12.1 × (WT/70)1.0 | 10.6 | |||
| Ka (h−1) | 2.38 | 17 | %CV = 53.3 | 36.8 | 49 |
| F (%) | 1.08 | 2 | |||
| Residual error, oral TPM | %CV = 18.4 | 21.8 | 7 | ||
| Residual error, IV TPM | %CV = 7.2 | 11.8 | 7 |
Abbreviations are as follows: CL, clearance; F, oral bioavailability of TPM; IV, intravenous; Ka, the first-order rate constant of absorption; Q, intercompartmental clearance; TPM, topiramate; Vc, central volume of distribution; Vp, peripheral volume of distribution; WT, bodyweight.
RSE, relative standard error = (standard error ÷ estimate) × 100. CV, coefficient of variation; 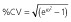 .
.
%RSE for random effects calculated in the variance scale.
