Table 3.
Parameter estimates of the pharmacokinetic–pharmacodynamic models
| Parameter | Analysis of COWA as continuous data | Poisson distribution for discrete data | ||
|---|---|---|---|---|
| Estimate | RSE (%)† | Estimate | RSE (%)† | |
| BL (words per three trials) | 42.5 | 3.48 | 42.4 | 3.51 |
| KE (l mg−1) | 0.157 | 6.97 | 0.163 | 16.7 |
| θN_COWA ≥ 4 | 1.12 | 2.38 | 1.13 | 2.35 |
| %CV for IIV of BL‡ | 17 [9] | 26.2 | 17.6 [6] | 25.7 |
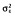 (words per three trials)
(words per three trials)
|
7.1 [8] | 15.4 | ||
| AIC | 829 | 1124 | ||
Abbreviations are as follows: AIC, Akaike information criterion; BL, baseline score of COWA; COWA, Controlled Oral Word Association; KE, the exponential decline constant for the effect of topiramate concentration; θN_COWA ≥ 4, estimate of the fractional increase in BL when four or more tests of COWA are given; estimates of percentage shrinkage are indicated in the square brackets [% shrinkage]; 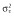 , variance of the residual error. †RSE, relative standard error = (standard error ÷ estimate) × 100; %RSE for random effects calculated in the variance scale. ‡IIV, interindividual variability; CV, coefficient of variation;
, variance of the residual error. †RSE, relative standard error = (standard error ÷ estimate) × 100; %RSE for random effects calculated in the variance scale. ‡IIV, interindividual variability; CV, coefficient of variation; 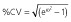 .
.
