Fig 6. The P618L substitution impairs the URT1 activity, affects its subcellular localization and diminishes its interaction with AGO1.
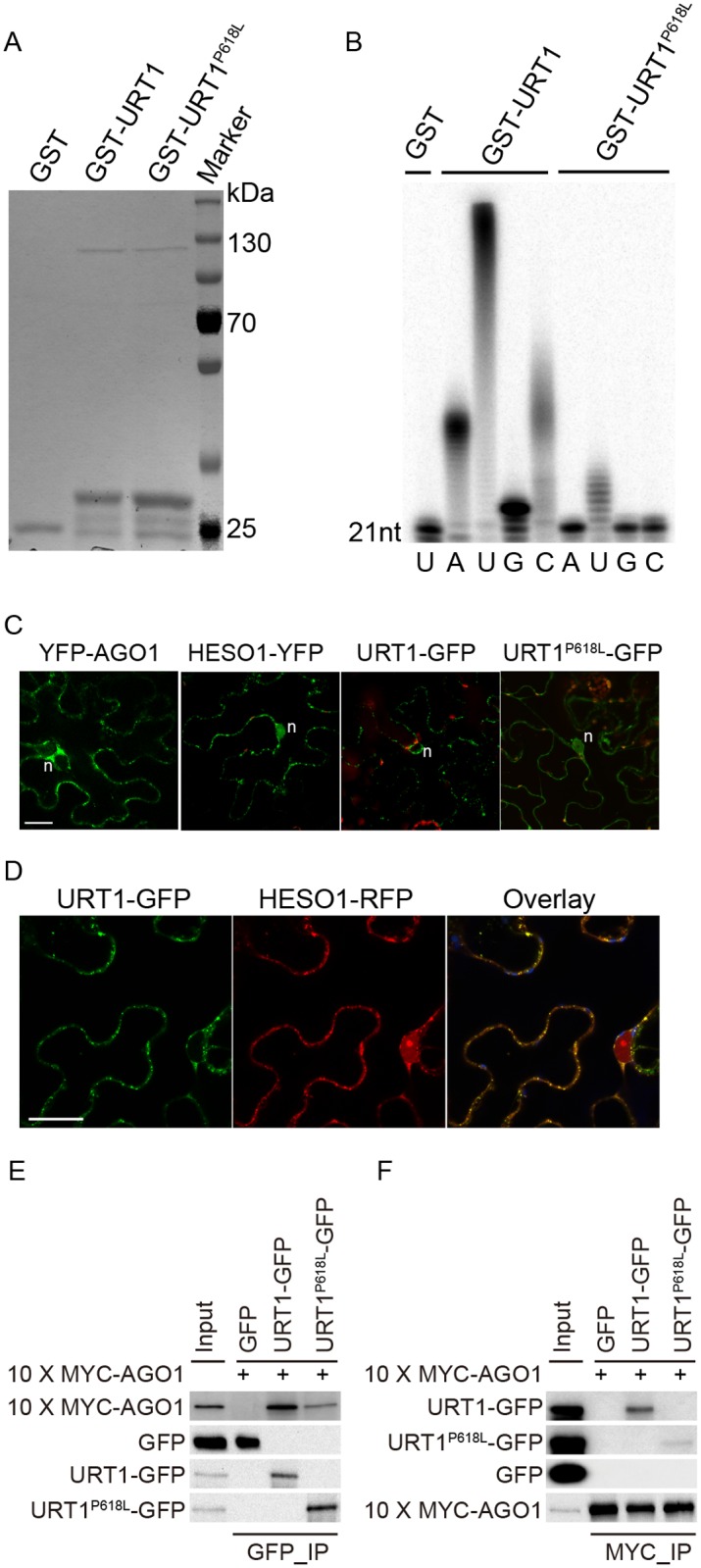
(A) Expression and purification of GST, GST-URT1 and GST-URT1P618L in E.coli BL21(DE3). After purification, proteins were subject to SDS-polyacrylamide gel analysis and monitored by the comassie brillant blue staining. (B) Terminal nucleotidyl transferase activity of URT1 and URT1P618L. 5’ end P32 labeled miR165a were incubated with GST, GST-URT1 or GST-URT1P618L in the presence of various nucleotide triphosphates for 30 minutes. After the reaction, RNAs were extracted and analyzed on a denaturing polyacrylamide gel. (C) Sub-cellular localization of YFP-AGO1, HESO1-YFP, URT1-GFP and URT1P618L-GFP. Note that only about 20% of YFP-AGO1 showed discrete cytoplasmic foci (C) while the remaining ones had a relatively even distribution[26]. For each construct, 40–50 cells were analyzed and a similar nucleus localization pattern was observed. n, nucleus. Scale bar, 20 μm. (D) URT1-GFP colocalized with HESO1-RFP. Paired constructs were coinfiltrated into N. benthamiana leaves. RFP and GFP fluorescence signals were monitored 40–48 h after infiltration by confocal microscopy. Scale bar, 20 μm. (E, F) Interactions between AGO1 and URT1 by the co-immunoprecipitation assay. 10xMYC-AGO1 and respective GFP tagged proteins were co-expressed in N. benthamiana leaves in the presence of P19 for 3 days and protein extracts were incubated with either anti-MYC-protein G-agarose beads or anti-GFP-protein G-agarose beads for 4 hrs to overnight. After reaction, proteins were resolved by the SDS-polyacrylamide gel electrophoresis and detected with respective antibodies. Input = 1%. (E) 10xMYC-AGO1 co-immunoprecipitates with URT1-GFP and URT1P618L-GFP. (F) URT1-GFP and URT1P618L-GFP co-immunoprecipitates with 10xMYC-AGO1.
