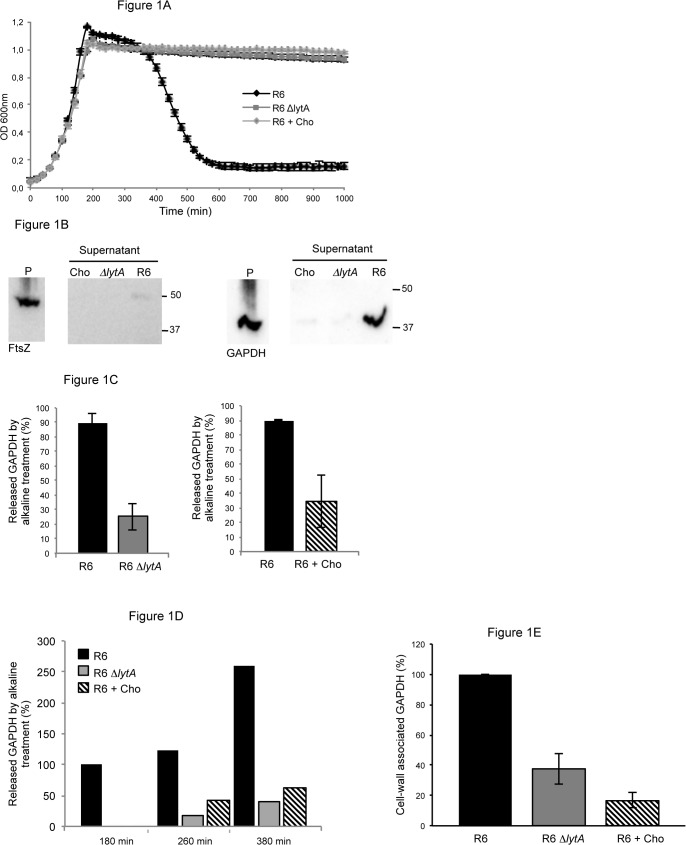
Fig 1. Pneumococcal lysis induced by LytA promotes GAPDH surface localization.
(A) Growth profiles of the R6 strain in CY and CY + 1% Cho and of the R6 ΔlytA mutant in CY medium. (B) Bacterial suspensions of the R6 strain grown in CY and in CY + 1% Cho and the R6 ΔlytA mutant grown in CY were treated by alkaline buffer to release surface-associated proteins. Proteins present in the pellet (P) fraction corresponding to the cytoplasmic extract and in the alkaline supernatant fraction, which contains proteins detached from the cell surface, were analyzed by Western blot using appropriate polyclonal antibodies. Samples were analyzed on the same polyacrylamide gel. Left panel: detection of FtsZ (44.4 kDa) used to monitor the non-lytic effect of the alkaline treatment. Right panel: detection of GAPDH (38 kDa). Equivalent amount of loading material was determined based on OD600nm values and gel scanning quantification and not on CFU measurements since the chaining morphology of the R6 ΔlytA mutant and the one induced by the presence of 1% Cho alters colony counting [65]. This procedure was also applied in experiments showed in Figs 1C, 1D and 1E. (C) Quantification of pneumococcal GAPDH associated to the bacterial surface in the R6 strain grown in CY and in CY + 1% Cho and in the R6 ΔlytA mutant grown in CY. GAPDH was detected by Western blot and quantification of the signal was performed. The average of three independent experiments is shown. (D) Same protocol as 1C. Amount of GAPDH associated to the cell wall fraction at different stages of growth. (E) Same protocol as 1C. Amount of GAPDH associated to the cell wall fraction analyzed by subcellular fractionation.