Abstract
The characterization of host immune responses to human immunodeficiency virus (HIV) in HIV controllers and individuals with high exposure but seronegativity to HIV (HESN) is needed to guide the development of effective preventive and therapeutic vaccine candidates. However, several technical hurdles severely limit the definition of an effective virus-specific T-cell response. By using a toggle-peptide approach, which takes HIV sequence diversity into account, and a novel, boosted cytokine staining/flow cytometry strategy, we here describe new patterns of T-cell responses to HIV that would be missed by standard assays. Importantly, this approach also allows detection of broad and strong virus-specific T-cell responses in HESN individuals that are characterized by a T-helper type 1 cytokine–like effector profile and produce cytokines that have been associated with potential control of HIV infection, including interleukin 10, interleukin 13, and interleukin 22. These results establish a novel approach to improve the current understanding of HIV-specific T-cell immunity and identify cellular immune responses and individual cytokines as potential markers of relative HIV resistance. As such, the findings also help develop similar strategies for more-comprehensive assessments of host immune responses to other human infections and immune-mediated disorders.
Keywords: boosted flow cytometry, highly exposed seronegative, HIV infection, toggled peptides, T-cell responses, Th1 cytokines, Th2 cytokines, Th17 cytokines
Understanding the characteristics of immune responses conferring relative protection from human immunodeficiency virus (HIV) infection or HIV disease progression is of great importance for the design of effective HIV vaccines. Identifying such immune parameters in individuals that remain seronegative despite recurrent HIV exposure could crucially guide preventive vaccine development. As the mechanisms associated with relative protection from HIV infection in subjects with high exposure but seronegativity to HIV (HESN) remain incompletely understood, concerted efforts are under way to better characterize immunological, virological, and host genetic markers that may protect such individuals from infection [1]. Some immune parameters and virus-specific immune responses that may mediate relative protection have been described [2–6], although technical issues and the inconsistent detection of virus-specific immunity in HESN individuals have spurred some controversy as to their physiological relevance [7–11].
In addition to HESN individuals, studies in HIV-infected individuals who can control HIV infection for years in the absence of antiretroviral treatment have identified several host factors that appear to mediate such relative control from disease progression [12, 13]. To date, the maintenance of polyfunctional, virus-specific CD8+ T-cell responses has been most consistently associated with reduced viral loads in chronic HIV infection [14, 15]. Furthermore, superior in vivo viral control has also been linked to cytotoxic T-lymphocyte responses of high functional avidity and broad variant-recognition potential, especially to variable segments of the viral genome [16, 17]. Such responses to more-variable segments may also be the ones that often escape in vitro detection, as monomorphic in vitro test sets do not cover sequence diversity appropriately [18]. In recent years, several approaches have been developed to cope with sequence diversity, both for vaccine design and for sensitive in vitro detection of virus-specific T-cell populations [19, 20]. Among them, the toggle-peptide approach has been shown to significantly increase in vitro detection rates of both HIV-specific CD4+ and CD8+ T-cell responses [21]. Toggle peptides are restricted, small peptide libraries that incorporate mixes of amino acids in the peptide synthesis for positions displaying sequence variability. This creates a limited set of peptide variants that cover sequence diversity up to a desired, a priori–defined cutoff and that, by definition, always include a sequence that represents the consensus sequence, as well [21].
Aside from sequence diversity, the limited set of effector functions that is assessed in most in vitro assays is another reason for our incomplete picture of virus-specific T-cell activities. Detection of interferon γ (IFN-γ) production by enzyme-linked immunosorbent (ELISpot) analysis is a sensitive approach for identifying antigen-specific T cells, and measurement of additional effector functions by intracellular cytokine staining (ICS) has produced reproducible associations with relative HIV control. However, the continuously expanding set of T-cell subpopulations with variable effector function profiles strongly suggest that these 2 standard assays will miss at least a portion of the cellular immune response. To overcome these limitations, we combined a novel, intracellular, boosted flow cytometry technique (hereafter, “boosted flow”) and toggle peptides to capture a more comprehensive picture of the T-cell immunity to HIV. The increased rate of detection of responses by boosted flow is achieved by combining several cytokines into the same fluorescence channel. This allows one to cover a vastly larger set of effector functions than afforded by standard assays, even if flow cytometry resources are limited to a few colors and fluorescence channels. The present data establish this combined strategy as a powerful tool to identify antigen-specific T-cell responses and specific cytokine patterns and effector function profiles in individuals who can control their HIV infection in the absence of antiretroviral treatment or who remain uninfected despite repeated exposure to HIV. Overall, these strategies allow for a more comprehensive determination of pathogen-specific T-cell immunity in general and may prove particularly helpful in detecting potentially nonclassical effector functions and responses in HESN individuals and, importantly, in subjects enrolled in HIV vaccine trials.
MATERIAL AND METHODS
Patients
Seventeen HIV-seropositive subjects (10 controllers and 7 noncontrollers), 8 HESN individuals, and 8 HIV-unexposed subjects were recruited at the Hospital Germans Trias i Pujol and the BCN Checkpoint community center for men who have sex with men, both of which are in Barcelona, Spain. Subjects with viral loads of <3500 copies/mL in the absence of antiretroviral treatment and CD4+ T-cell counts >300 cells/mm3 for the past 3 years or more were considered HIV controllers (median viral load, 995 copies/mL [range, <25–3500 copies/mL]; median CD4+ T-cell count, 657 cells/mm3 [range, 310–1732 cells/mm3]). The noncontrollers were defined as highly active antiretroviral therapy–naive individuals with a plasma HIV RNA load of > 50 000 copies/mL (median, 210 000 copies/mL [range, 83 000–720 000 copies/mL]) and a CD4+ T-cell count of <300 cells/mm3 (median, 119 cells/mm3 [range, 6–296 cells/mm3]; Table 1). HESN individuals were identified from a high-risk cohort of men who have sex with men who are followed up every 3 months at BCN Checkpoint. The criteria for inclusion of HESN were >1 unprotected episode of sexual contact during the previous 3 months with a HIV seropositive individual and >50 different partners/year. The prevalence of HIV infection in this population is around 20% [22]. The tested HESN individuals included 3 subjects who were identified and enrolled upon receipt of a first-time diagnosis of HIV infection by sex partners with whom they had engaged in unprotected sex during the months leading up to the diagnosis. The study was approved by the local research ethics committees, and all participants provided written informed consent.
Table 1.
Viral Load and CD4+ T-Cell Count Among Human Immunodeficiency Virus (HIV)–Seropositive Study Subjects
| Group, Patient | Viral Load, Copies/mL | CD4+ T-Cell Count, Cells/mm3 |
|---|---|---|
| Controllers | ||
| C1 | 2800 | 671 |
| C2 | 1000 | 1205 |
| C3 | 400 | 482 |
| C4 | 630 | 1732 |
| C5 | 990 | 310 |
| C6 | <25 | 727 |
| C7 | <25 | 643 |
| C8 | 400 | 610 |
| C9 | 3500 | 597 |
| C10 | 1500 | 741 |
| Noncontrollers | ||
| NC1 | 83 000 | 119 |
| NC2 | 510 000 | 6 |
| NC3 | 720 000 | 15 |
| NC4 | 210 000 | 85 |
| NC5 | 680 000 | 171 |
| NC6 | 85 000 | 259 |
| NC7 | 170 000 | 296 |
Values for healthy donors and individuals with high exposure but seronegativity to HIV were not determined.
Cell Culture
For all assays, freshly isolated peripheral blood mononuclear cells (PBMCs) were used. For flow cytometry and FlowCytomix studies, 500 000 cells/well were added to 48-well flat-bottomed plates in Roswell Park Memorial Institute medium for 6 hours, for ICS assays, or for 5 days, for detection of cytokines in supernatant. Cells were stimulated with p24 toggled peptides (Table 2) at a final concentration of 10 µg/mL. Anti-CD3 plus anti-CD28 magnetic beads (Invitrogen), PMA plus ionomycin, or Epstein-Barr virus peptide pools (10 µg/mL [23]) were used as positive controls.
Table 2.
Toggle Peptides and Their Consensus Overlapping Sequences (OLPs)
| Peptide | Sequence | Variants, No. | Consensus OLP |
|---|---|---|---|
| TV12-17 | AA[DG]TG[NS][SN]SQVSQNYPIV | 8 | AADTGNSSQVSQNYPIV |
| TV12-18 | [SN][QP]VSQNYPIVQN[LIM]QGQMV | 12 | SQVSQNYPIVQNLQGQMV |
| TV12-19 | IVQN[LIM]QGQMVHQ[AP][IL]SPR | 12 | IVQNLQGQMVHQAISPR |
| TV12-20 | QMVHQ[APS][IL]SPRTLNAWVKV | 6 | QMVHQAISPRTLNAWVKV |
| TV12-21 | PRTLNAWVKV[VI]EEKAF | 2 | PRTLNAWVKVVEEKAF |
| TV12-22 | WVKV[VI]EEKAF[SN]PEVIPMF | 4 | WVKVVEEKAFSPEVIPMF |
| TV12-23 | AFSPEVIPMF[STA]AL[SA]EGA | 6 | AFSPEVIPMFSALSEGA |
| TV12-24 | PMF[ST]AL[SA]EGATPQDLNTM | 4 | PMFSALSEGATPQDLNTM |
| TV12-25 | GATP[QS]DLNTMLNTVGGH | 2 | GATPQDLNTMLNTVGGH |
| TV12-26 | NTMLNTVGGHQAAMQ[MI]LK | 2 | NTMLNTVGGHQAAMQMLK |
| TV12-27 | GGHQAAMQMLK[ED]TINEEA | 2 | GGHQAAMQMLKETINEEA |
| TV12-28 | LK[ED]TINEEAA[ED]WDR[LV]HPV | 8 | LKETINEEAAEWDRLHPV |
| TV12-29 | AAEWDR[LV]HPV[HQ]AGP[IV]A | 8 | AAEWDRLHPVHAGPIA |
| TV12-30 | LHPV[HQ]AGP[IV]APGQ[MI]REPR | 8 | LHPVHAGPIAPGQMREPR |
| TV12-31 | [IV]APGQ[MI]R[ED]PRGSDIA | 8 | IAPGQMREPRGSDIA |
| TV12-32 | [MI]R[ED]PRGSDIAGTTS[TN]L | 8 | MREPRGSDIAGTTSTL |
| TV12-33 | SDIAGTTS[TN]LQEQI[GATQ]WM | 8 | SDIAGTTSTLQEQIGWM |
| TV12-34 | S[TN]LQEQI[GA]WMT[NSH]NPPIPV | 12 | STLQEQIGWMTNNPPIPV |
| TV12-35 | WMT[NSH]NPP[IV]PVG[ED]IYKRWI | 12 | WMTNNPPIPVGEIYKRWI |
| TV12-36 | PVG[ED]IYKRWII[LM]GLNKIV | 4 | PVGEIYKRWIILGLNKIV |
| TV12-37 | WII[LM]GLNKIVRMYSP[TVI]SI | 6 | WIILGLNKIVRMYSPTSI |
| TV12-38 | IVRMYSP[TVIS]SILDI[RK]QGPK | 8 | IVRMYSPTSILDIRQGPK |
| TV12-39 | SILDI[RK]QGPKEPFRDYV | 2 | SILDIRQGPKEPFRDYV |
| TV12-40 | GPKEPFRDYVDRFYK[TV]LR | 2 | GPKEPFRDYVDRFYKTLR |
| TV12-41 | YVDRFYK[TV]LRAEQA[ST]Q[ED]V | 8 | YVDRFYKTLRAEQASQEV |
| TV12-42 | LRAEQA[ST]Q[ED]VK[NG]WMTETL | 8 | LRAEQASQEVKNWMTETL |
| TV12-43 | [ED]VK[NGH]WMTETLLVQN[AS] | 12 | EVKNWMTETLLVQNA |
| TV12-44 | MTETLLVQN[AS]NPDCKTIL | 2 | MTETLLVQNANPDCKTIL |
| TV12-45 | N[AS]NPDC[KR]TILKALGP[AG]A | 8 | NANPDCKTILKALGPAA |
| TV12-46 | TILKALGP[AG]A[TS]LEEMMTA | 4 | TILKALGPAATLEEMMTA |
| TV12-47 | [AG]A[TS]LEEMMTACQGVGGP[GS]H | 8 | AATLEEMMTACQGVGGPGH |
FlowCytomix Analyses
In culture supernatants harvested after 5 days, 13 cytokines were detected using a Th1/Th2/Th9/Th17/Th22 13-plex FlowCytomix kit (eBioscience) in accordance with the manufacturer's instructions.
Flow Cytometry
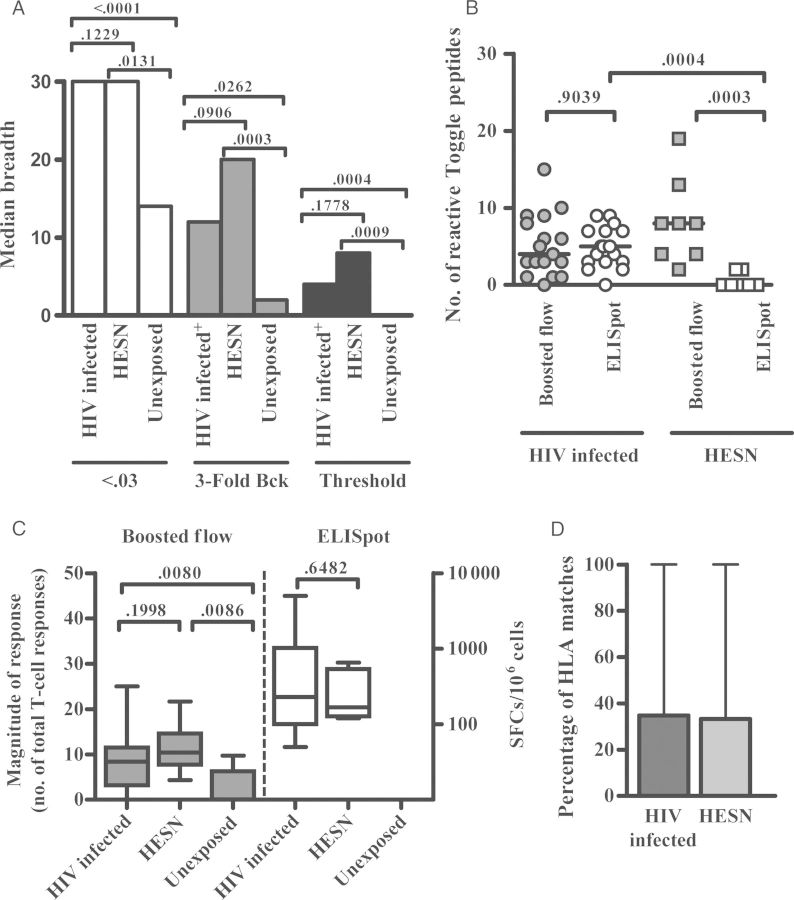
Flow cytometry for cytokine detection was performed as described previously by Lamoreaux et al [24], with small modifications for boosted flow. Briefly, PBMCs were stimulated with CD49d and CD28 antibodies (Becton Dickinson, Mountain View, California) and toggled peptides in the presence of GolgiStop, and cultures were then stored overnight at 4°C until staining. Viability staining (Live/Dead Fixable Dead Cell Stain kit, Invitrogen) was performed first, followed by staining for T-cell markers (using CD3-PE, CD4-APC, and CD8-V500; Becton Dickinson) and staining for exclusion of B lymphocytes and myeloid cells (using CD19-V450 and CD14-V450; Becton Dickinson). Following the fixation and permeabilization step (Fix and Perm kit, Invitrogen), intracellular staining with antibodies against Th1 cytokines conjugated with FITC (for IFN-γ, interleukin 2 [IL-2], tumor necrosis factor α [TNF-α], and MIP-1β; Becton Dickinson) and with antibodies against Th2/17 cytokines conjugated with PE-Cy7 (for interleukin 4 [IL-4; eBioscience], interleukin 10 [IL-10; Biolegend], and interleukin 17 [IL-17; eBioscience]) was performed. Cells were collected on an LSR II instrument (Becton Dickinson), and T-cell cytokine production analysis was performed using FACSDiva and FlowJo 7.6.5 software (the gating strategy is summarized in Supplementary Figure 1). The percentage of background activity detected in unstimulated control cultures was subtracted from the magnitude of response seen in antigen-stimulated cultures. As conservative cutoff, the maximal negative value after background subtraction across all individuals and all toggle stimulations was taken to define the threshold for a positive response, as described by Roederer et al [25]. Figure 2A indicates how this cutoff compared with other, regularly used cutoffs for ICS, including cutoffs based on positive responses exceeding T-cell responses by 0.03% or the background activity by 3-fold. The threshold cutoff approach used in the present report represents the most stringent of all 3 approaches.
Figure 2.
Boosted flow and interferon γ (IFN-γ) enzyme-linked immunosorbent assay (ELISpot) responses against toggle peptides in human immunodeficiency virus (HIV)-infected subjects and individuals with high exposure but seronegativity to HIV (HESN). Freshly isolated peripheral blood mononuclear cells were stimulated with individual toggle peptides spanning the HIV Gag p24 consensus B sequence. Boosted flow and ELISpot analyses were conducted during 5-hour and 16-hour incubation periods, respectively. A, The median breadths of T-cell responses in 17 HIV-infected people, 8 HESN individuals, and 8 HIV-unexposed subjects after applying cutoffs based on positive responses exceeding T-cell responses by 0.03% (white), positive responses exceeding the background activity (Bck) by 3-fold (dark grey), and the threshold value, determined as described in Methods (black). B, The number of positive responses upon toggle-peptide stimulations detected using boosted flow (grey) or ELISpot (white) in 17 HIV-infected people (circles) and 8 HESN individuals (squares). Horizontal lines indicate the median of number of toggle proteins eliciting a positive response. C, The median magnitude of T-cell responses detected using boosted flow (left; grey) or ELISpot (right; white) in 17 HIV-infected patients, 8 HESN individuals, and 8 HIV-unexposed donors. Whiskers denote ranges, and tops and bottoms of boxes denote interquartile ranges. D, Median percentages of matches between the HLA types in subjects and the HLA restriction of the optimal epitope contained in the toggle peptide. Ranges are denoted by whiskers. Response rates between groups were compared by the Mann–Whitney test. Abbreviation: SFC, spot-forming cell.
IFN-γ ELISpot
Freshly isolated PBMCs (100 000 PBMCs/well) were stimulated with p24 toggled peptides. The IFN-γ Mabtech kit was used in accordance with the manufacturer's instructions [21]. The threshold for positive responses was defined as responses with more than 5 spots/well and responses exceeding the “mean number of spots in negative control wells plus 3 standard deviations of the negative control wells” and “three times the mean of negative control wells,” whichever was higher.
Statistical Analysis
Statistical analyses were performed using Prism, version 4 (GraphPad). χ2 analysis was used for detection of qualitative differences between the cytokine response patterns. For comparisons between patient groups, nonparametric Mann–Whitney test was used. In all the analysis, P values of <.05 were considered statistically significant.
RESULTS
Intracellular Boosted Flow Allows Detection of Broader T-Cell Responses
To overcome current limitations of assays that investigate single effector function, we developed and tested the novel strategy referred to as boosted flow. To not limit the detection of responses to those effector profiles normally assessed, we designed a staining assay that involves simultaneous detection of several cytokines in the same fluorescence channel. For this first study, we opted to combine Th1-like cytokines (IFN-γ, TNF-α, MIP-1β, and IL-2) in one panel and to merge Th2-like cytokines (IL-4 and IL-10) and the dominant cytokine characterizing Th17 cells (IL-17) in a second panel. To establish the assay, fresh PBMCs from HIV-unexposed healthy donors were stimulated with different positive controls and analyzed for the presence of T cells showing signals in either of the 2 cytokine channels. As shown in Figure 1A and 1B and Supplementary Figure 2, the mean fluorescence intensity (MFI) spectrum of the signal obtained with the combined cytokine stains (ie, boosted flow) reflected the MFI spectrum of the signals from the individual single-cytokine staining analyses. Not unexpectedly, the magnitudes for boosted signals were generally higher than the individual magnitudes detected by single ICS (Figure 1C), likely because cells did not simultaneously produce all of the investigated cytokines. However, when the signals for single cytokines were added and compared to the magnitudes yielded by boosted flow, no statistically significant differences were observed (Figure 1D), demonstrating that combined detection of different cytokine signals in 1 fluorescence channel is a sensitive approach for capturing multiple effector functions simultaneously.
Figure 1.
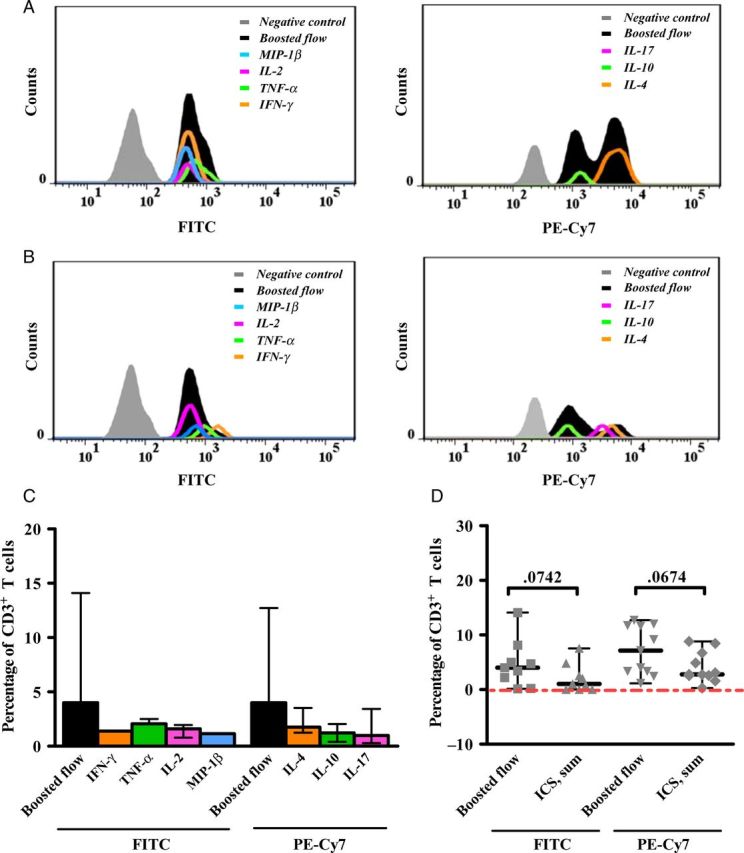
Results of single-cytokine intracellular staining assays and boosted flow for human immunodeficiency virus (HIV)-uninfected subjects. Freshly isolated peripheral blood mononuclear cells were stimulated with either CD3/CD28 magnetic beads, PMA plus ionomycin, or Epstein-Barr virus peptide pools, and intracellular cytokine staining (ICS) and boosted-flow signals were compared. Representative histograms of mean fluorescence intensities after FITC (left) and PE-Cy7 (right) staining for individual cytokines measured by single ICS assays (colors) and by boosted flow (black) are shown after polyclonal stimulation with PMA plus ionomycin (A) or upon T-cell–specific activation through anti-CD3 and anti-CD28 (B). The magnitude of antigen-specific responses detected by boosted flow was compared with respective single-cytokine levels (C) or summed magnitudes (D). Assays are shown for 4 uninfected subjects upon 3 positive stimulations. Medians of magnitudes with ranges (C and D) are represented and compared by the Wilcoxon signed rank test. Abbreviations: IFN-γ, interferon γ; IL, interleukin; TNF-α, tumor necrosis factor α.
Broader Detection of HIV-Specific T-Cell Responses Combining Toggle-Peptide Analysis and Boosted Flow
Toggle peptides have been shown to significantly increase response rates, compared with consensus or other centralized sequences [21, 26]. We thus combined boosted flow with toggle-peptide stimulations to test whether this strategy could detect responses in HIV-infected and, in particular, HESN individuals. A total of 30 overlapping toggle peptides were synthesized that cover the p24 region of HIV Gag [27, 28], since its relative conservation allows the synthesis of toggle peptides that cover the vast majority of sequence variants, including minor variants that may have stimulated T-cell responses in HESN individuals (Table 2). Toggle peptides were then used to stimulate PBMCs from 17 HIV-infected patients, 8 HESN individuals, and 8 healthy unexposed blood donors. In line with reports by others, responses detected by IFN-γ ELISpot were mainly confined to the group of 17 HIV-infected individuals, as only 2 of 8 HESN individuals showed a reactivity to 2 of 30 toggle peptides tested (Figure 2B). No responses were detected in 8 unexposed healthy donors by IFN-γ ELISpot. The few responses seen in HESN individuals were of comparable magnitude (median, 168 spot-forming cells [SFCs]/106 PBMCs) to responses detected in HIV-infected subjects (median, 230 SFCs/106 PBMCs; Figure 2C), suggesting that the responses in the HESN individuals were infrequent but not just borderline-positive signals.
Use of boosted flow and application of the threshold cutoff (Figure 2A) significantly increased the detection of responses in the HESN group, as all tested individuals mounted at least 2 positive T-cell responses (range, 2–20 responses/individual [median, 8 responses/individual]), compared with rare detection of responses in these individuals by the ELISpot assay (Figure 2B). The breadth of responses detected by boosted flow among HIV-infected subjects was comparable to ELISpot results (Figure 2B), suggesting that boosted flow reproducibly detected responses revealed by the ELISpot assay. Furthermore, the median magnitude of positive responses in HESN individuals and HIV-infected subjects was not different (11.5% and 7.17% of reactive T cells, respectively; Figure 2C), indicating that responses in the HESN group were as robust as those in HIV-infected subjects and were not due to insufficiently stringent cutoff definitions. Since boosted flow captures multiple effector functions in a single channel, commonly used cutoffs, such as positive responses exceeding the negative control for instance by 0.03% responding T cells or total T cells exceeding the negative background 3-fold, are not sufficiently stringent in this setting (see Methods; Figure 2A). In the HIV-unexposed controls, 1 response each was detected in 2 of 8 subjects (after 240 toggle-peptide stimulations; Figure 2B). Such occasional positive responses surpassing the stringent cutoff used for boosted flow are in line with recent data demonstrating the presence of HIV-reactive T cells even in unexposed individuals [29, 30].
To further validate that the boosted flow assay detected HIV-specific responses, the HLA types and toggle-peptide reactivities for each individual were compared to the location and HLA restriction sites of known optimal HIV epitopes [31]. For each individual, the number of responses to 18mer toggle peptides that contained an optimal epitope for which the individual expressed the restricting HLA allele was determined. Responses in HESN individuals matched a described optimal epitope in 33% of cases, while 35% of responses in HIV-infected subjects were explained by the presence of an optimal epitope (P > .5; Figure 2D). Overall, these data are in line with earlier studies that have reported infrequent or absent T-cell responses in ELISpot analyses involving HIV-exposed, uninfected subjects [7, 32] and suggest that (1) HESN individuals mount either fewer, weaker, or functionally different T-cell responses to HIV than chronically infected individuals and (2) that common in vitro tests may not adequately capture broader responses in HESN individuals.
Toggle Peptides Stimulate Responses With Different Cytokines Patterns Between HIV-Infected Patients and HESN Individuals
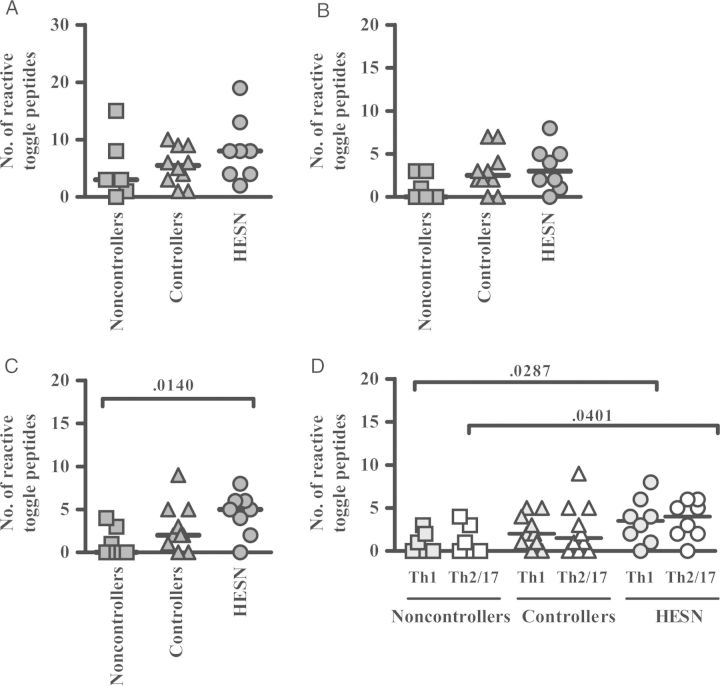
Next, the differential cytokine response patterns upon toggle-peptide stimulation were analyzed in the 8 HESN individuals and compared to those for HIV-infected patients. Since differences in effector function profiles have been described for HIV controllers and noncontrollers [14], the 17 HIV-infected subjects were divided into 10 controllers and 7 noncontrollers. Based on total numbers of CD4+ and CD8+ T cells, the breadth of responses detected by boosted flow in HESN individuals and HIV controllers tended to be greater than in noncontrollers (median, 3 for noncontrollers, 5.5 for controllers, and 8 for HESN individuals; Figure 3A). This increase was especially driven by CD8+ T cells, with levels significantly higher in HESN individuals (median, 5), compared with noncontrollers (median, 0; Figure 3C). Of note, these CD8+ T-cell responses in HESN individuals were significantly elevated in responses with a Th1-like cytokine expression level (Figure 3D) and resembled responses in HIV controllers.
Figure 3.
Increased detection of human immunodeficiency virus (HIV)–specific responses in individuals with high exposure but seronegativity to HIV (HESN), using boosted flow. Response rates associated with 30 toggle peptides covering HIV Gag p24 are shown for total T cells (A; dark grey) for 7 HIV noncontrollers (squares), 10 HIV controllers (triangles), and 8 HESN individuals (circles). Responses are further stratified into CD4+ T cells (B; dark grey) and CD8+ T cells (C; dark grey). D, CD8+ T-cell responses in either the T-helper type 1 (Th1)–like (light grey) or Th2/17-like (white) cytokine channel are shown. Horizontal bars indicate median numbers of toggle peptides eliciting a positive response. Response rates between groups were compared by the Mann–Whitney test.
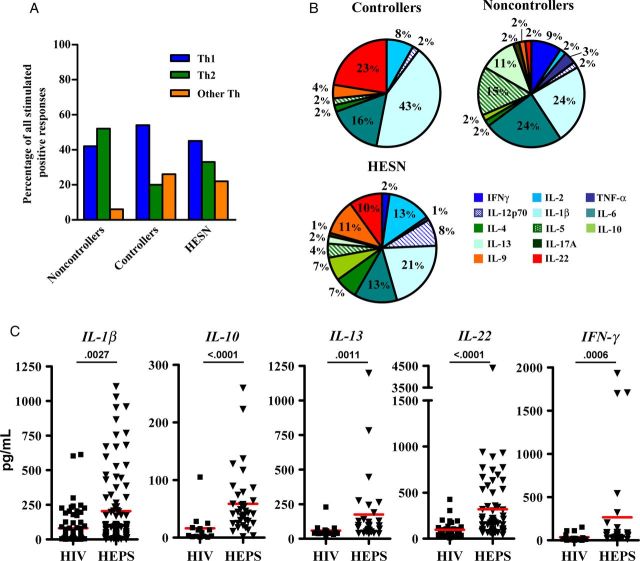
To gain further insight into the different response patterns in HIV-infected patients and HESN individuals and to identify cytokines that may be involved in protection from HIV infection or disease progression and thus could be included in future boosted flow panels, 13 different cytokines were measured in the supernatant after 5-day culture of toggle-peptide–stimulated PBMCs. These included the classical Th1 cytokines (IFN-γ, TNF-α, interleukin 1β [IL-1β], IL-2, and interleukin 12p70) and Th2-like cytokines (IL-4, interleukin 5, interleukin 6 [IL-6], IL-10, and interleukin 13 [IL-13]), plus cytokines attributed to Th9 (interleukin 9), Th17 (IL-17), and Th22 (interleukin 22 [IL-22]) T-cell responses. Cultures of specimens from HIV controllers scored positive for Th1 cytokines more often than cultures of specimens from noncontrollers (χ2 = 13.53; P < .0001; Figure 4A). Similar to HESN individuals, controllers showed production of cytokines associated with other Th-like responses (including Th9, Th17, and Th22 responses) more often than noncontrollers (χ2 = 13.49; P = .0012; Figure 4A and Figure 4B). Concentrations of IL-1β, IL-10, IL-13, IL-22, and IFN-γ in HIV-infected individuals were statistically significantly different from concentrations in HESN individuals (Figure 4C). In addition, concentrations of IL-6 and IL-1β were elevated in HESN individuals, compared with those in HIV noncontrollers (Supplementary Figure 3). These analyses thus identify specific cytokine patterns that are either uniquely induced upon in vitro toggle-peptide stimulation in HESN individuals or that may represent differences in underlying constitutive cytokine production in HESN individuals that could be involved in relative resistance to HIV acquisition. Importantly, they also identify candidate cytokines that may be included in future refined boosted-flow panels, especially when testing HESN individuals or vaccine recipients.
Figure 4.
Detection of 13 released cytokines in culture supernatants of peripheral blood mononuclear cells stimulated with toggle peptides. Supernatants of 1-week cultures were assessed for T-helper type 1 (Th1)–like cytokines (interferon γ [IFN-γ], tumor necrosis factor α [TNF-α], interleukin 1β [IL-1β], interleukin 2 [IL-2], and interleukin 12p70 [IL-12p70]), Th2-like cytokines (interleukin 4 [IL-4], interleukin 5 [IL-5], interleukin 6 [IL-6], interleukin 10 [IL-10], and interleukin 13 [IL-13]), and interleukin 17A (IL-17A), interleukin 9 (IL-9), and interleukin 22 (IL-22). The latter 3 cytokines are grouped in panel A as “other Th.” The number of positive responses in each panel is given as the percentage of all stimulated wells. B, Distribution of cytokine signals among all positive responses in 8 individuals with high exposure but seronegativity to human immunodeficiency virus (HIV; HESN), 10 HIV controllers, and 7 noncontrollers. C, Individual cytokine levels in 17 HIV-infected individuals and 8 HESN individuals. P values are from comparisons by the Mann–Whitney test. Horizontal lines indicate median of cytokine levels (pg/ml) per patient and stimulation.
DISCUSSION
Understanding the characteristics of immune responses that might control HIV infection or defend against acquisition of HIV will greatly help development of future HIV vaccines. We tested HIV-infected patients and HESN individuals with a newly developed approach for measuring HIV-specific T-cell responses that is able to simultaneously detect various Th1, Th2, and Th17 cytokines in different T-cell subsets. The combined use of toggled peptides in boosted flow, a novel flow cytometry assay, and subsequent detection of cytokines in culture supernatants provide strong evidence that T-cell responses to HIV can be detected in HESN individuals if sensitive tests are used and that these responses are robust, antigen specific, and thus potentially physiologically relevant.
An apparent concern about boosted flow may be its potential for high background staining, as all the cytokines included in a specific panel will contribute additively to the overall signal. This may however also give boosted flow the power to detect weaker responses that would rank below the detection limit for each individual effector function alone or to identify responses that produce cytokines that are not captured by standard cytokine assays. In addition, a portion of the detected signal may derive from non–antigen-specific T cells that undergo bystander activation and that help amplify the antigen-specific signal into detectable ranges. Along with stringent cutoffs for positive responses, these factors may provide boosted flow with the required robustness and sensitivity to identify responses in HESN individuals.
It is also possible that some antigen-unspecific stimulation occurs because of cross-reactivity between toggle-peptide sequences and epitopes derived from other copathogens and self-proteins [33]. However, this appears to be less likely, because we did not observe a relationship between the number of variants in the toggle-peptide mix and the frequency at which it induced in vitro reactivity. Moreover, the relative absence of responses in HIV-unexposed individuals and the comparable percentage of HLA/optimal epitope matches in HIV-infected individuals and HESN subjects further suggest that responses were HIV specific. Of note, in the present study, we combined Th2-like and Th17-like responses in 1 channel, with the awareness that these cytokines may not be produced by the same cells. This design reflects a compromise between occupying fewer channels in the flow cytometer and casting a wide net to identify possible responses in HESN individuals. Although different future applications will call for alternative composition and/or larger numbers of panels (such as those that include IL-13 and IL-22), the combinations used here allowed the detection of responses in HESN individuals that would have gone undetected by other current techniques.
Finally, comparison of data yielded by boosted flow with those from supernatant analysis is complicated by the fact that cytokines may be consumed over time or may be produced by non–T-cell populations. This could possibly yield additional signals of the innate immune response, including the reportedly elevated production of IL-1β, IL-6, TNF-α, and CCL3 via Toll-like receptor 3 (TLR-3), TLR-4, and TLR-7/8 signaling in HESN individuals [34]. Yet such analyses can help identify cytokines with constitutively higher expression in HESN individuals, compared with HIV-infected individuals, as well as cytokines that may characterize potentially protective HIV-specific cellular immune responses in HESN individuals. In this regard, it is intriguing that IL-10 and IL-13, which showed higher levels in HESN individuals, have also emerged from recent posttrial analyses of the RV144 HIV vaccine trial as markers of vaccine-mediated protection. These cytokines should be included in future boosted-flow panels to elucidate whether they are HIV-unspecific factors that predisposed vaccine recipients to relative protection from HIV infection or whether they are associated with antigen-specific protective effects of vaccine-induced immune responses [35]. Similar to IL-10 and IL-13, IL-22 was highly overexpressed in HESN individuals, compared with HIV-infected individuals, in line with its recently described potentially protective role in HESN individuals [1, 36], and offers another candidate for future refined boosted-flow panel design.
Together, the present results establish boosted flow as a powerful approach to identify and characterize HIV-specific T-cell responses in HESN individuals. Our data and recent studies describing HIV-reactive responses even in HIV-unexposed subjects provide further strong support that T-cell responses in HESN individuals are not just borderline activities without potential in vivo relevance. Yet it remains to be clarified whether the different cytokine profiles in HESN individuals and chronically HIV-infected subjects reflect a protective antiviral activity, not just healthier T cells, in HESN individuals, compared with more-exhausted T cells in HIV-infected patients. Nevertheless, the data may provide critical clues for the development of preventive and therapeutic immune-based interventions for HIV infection. In the face of ever-expanding sets of markers that define distinct T-cell subsets and differentiation stages (be it in healthy individuals, subjects infected with HIV or any other pathogen, and subjects with autoimmune diseases), the ability to functionally capture these cells will be crucial for a better understanding of their in vivo role and destiny.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the patients and the noninfected study subjects who participated in this research.
Financial support. This work was supported by the HIVACAT program, the FIS (grants PS0900283 and FIPSE36-0737-09 to C. B.), the European Community (CUT'HIVAC; grant EC-7FP-241904 to C. B.), the National Institutes of Health (grant R01 DE018925-05 to C. B.), the ISCIII (Rio Hortega; grants CM08/00020 and JR13/00024 to B. M.), the Institució Catalana de Recerca I Estudis Avançats (to C. B.), an unrestricted donation by Rafael Punter, and the Barcelona HIV Gala 2012.
Potential conflicts of interest. A patent application describing boosted flow has been submitted (PCT/EP2013/056110). The authors do not report any other potential conflict of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Burgener A, Sainsbury J, Plummer FA, Ball TB. Systems biology-based approaches to understand HIV-exposed uninfected women. Curr HIV/AIDS Rep. 2010;7:53–9. doi: 10.1007/s11904-010-0039-3. [DOI] [PubMed] [Google Scholar]
- 2.Erickson AL, Willberg CB, McMahan V, et al. Potentially exposed but uninfected individuals produce cytotoxic and polyfunctional human immunodeficiency virus type 1-specific CD8(+) T-cell responses which can be defined to the epitope level. Clin Vaccine Immunol. 2008;15:1745–8. doi: 10.1128/CVI.00247-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goh WC, Markee J, Akridge RE, et al. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis. 1999;179:548–57. doi: 10.1086/314632. [DOI] [PubMed] [Google Scholar]
- 4.Hasselrot K, Saberg P, Hirbod T, et al. Oral HIV-exposure elicits mucosal HIV-neutralizing antibodies in uninfected men who have sex with men. AIDS. 2009;23:329–33. doi: 10.1097/QAD.0b013e32831f924c. [DOI] [PubMed] [Google Scholar]
- 5.Kaul R, Plummer FA, Kimani J, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–11. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 6.Schmechel SC, Russell N, Hladik F, et al. Immune defence against HIV-1 infection in HIV-1-exposed seronegative persons. Immunol Lett. 2001;79:21–7. doi: 10.1016/s0165-2478(01)00262-0. [DOI] [PubMed] [Google Scholar]
- 7.Addo MM, Altfeld M, Brainard DM, et al. Lack of detectable HIV-1-specific CD8(+) T cell responses in Zambian HIV-1-exposed seronegative partners of HIV-1-positive individuals. J Infect Dis. 2011;203:258–62. doi: 10.1093/infdis/jiq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begaud E, Chartier L, Marechal V, et al. Reduced CD4T cell activation and in-vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis. 2009;199:1318–22. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- 10.Hladik F, Desbien A, Lang J, et al. Most highly exposed seronegative men lack HIV-1-specific, IFN-gamma-secreting T cells. J Immunol. 2003;171:2671–83. doi: 10.4049/jimmunol.171.5.2671. [DOI] [PubMed] [Google Scholar]
- 11.Koning FA, Otto SA, Hazenberg MD, et al. Low-level CD4+ T cell activation is associated with low susceptibility to HIV-1 infection. J Immunol. 2005;175:6117–22. doi: 10.4049/jimmunol.175.9.6117. [DOI] [PubMed] [Google Scholar]
- 12.Mothe B, Ibarrondo J, Llano A, Brander C. Virological, immune and host genetics markers in the control of HIV infection. Dis Markers. 2009;27:105–20. doi: 10.3233/DMA-2009-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereyra F, Jia X, McLaren PJ, et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–7. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Migueles SA, Tilton JC, Connors M. Qualitative host factors associated with immunological control of HIV infection by CD8T cells. Curr Opin HIV AIDS. 2006;1:28–33. doi: 10.1097/01.COH.0000194108.14601.69. [DOI] [PubMed] [Google Scholar]
- 16.Mothe B, Llano A, Ibarrondo J, et al. Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med. 2011;9:208. doi: 10.1186/1479-5876-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mothe B, Llano A, Ibarrondo J, et al. CTL responses of high functional avidity and broad variant cross-reactivity are associated with HIV control. PLoS One. 2012;7:e29717. doi: 10.1371/journal.pone.0029717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frahm N, Korber BT, Adams CM, et al. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J Virol. 2004;78:2187–200. doi: 10.1128/JVI.78.5.2187-2200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brander C, Frahm N, Walker BD. The challenges of host and viral diversity in HIV vaccine design. Curr Opin Immunol. 2006;18:430–7. doi: 10.1016/j.coi.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Brander C, Self S, Korber B. Capturing viral diversity for in-vitro test reagents and HIV vaccine immunogen design. Curr Opin HIV AIDS. 2007;2:183–8. doi: 10.1097/COH.0b013e3280f3bfe2. [DOI] [PubMed] [Google Scholar]
- 21.Frahm N, Kaufmann DE, Yusim K, et al. Increased sequence diversity coverage improves detection of HIV-specific T cell responses. J Immunol. 2007;179:6638–50. doi: 10.4049/jimmunol.179.10.6638. [DOI] [PubMed] [Google Scholar]
- 22.Mackelprang RD, Baeten JM, Donnell D, et al. Quantifying ongoing HIV-1 exposure in HIV-1-serodiscordant couples to identify individuals with potential host resistance to HIV-1. J Infect Dis. 2012;206:1299–308. doi: 10.1093/infdis/jis480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bihl F, Frahm N, Di Giammarino L, et al. Impact of HLA-B alleles, epitope binding affinity, functional avidity, and viral coinfection on the immunodominance of virus-specific CTL responses. J Immunol. 2006;176:4094–101. doi: 10.4049/jimmunol.176.7.4094. [DOI] [PubMed] [Google Scholar]
- 24.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507–16. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 25.Roederer M, Nozzi JL, Nason MX. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 2011;79:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frahm N, Nickle DC, Linde CH, et al. Increased detection of HIV-specific T cell responses by combination of central sequences with comparable immunogenicity. AIDS. 2008;22:447–56. doi: 10.1097/QAD.0b013e3282f42412. [DOI] [PubMed] [Google Scholar]
- 27.Kiepiela P, Ngumbela K, Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 28.Zuniga R, Lucchetti A, Galvan P, et al. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol. 2006;80:3122–5. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campion SL, Brodie TM, Fischer W, et al. Proteome-wide analysis of HIV-specific naive and memory CD4+ T cells in unexposed blood donors. J Exp Med. 2014;211:1273–80. doi: 10.1084/jem.20130555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–83. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Llano A. How to optimally define optimal cytotoxic T lymphocyte epitopes in HIV infection? In: Korber B, Brander C, Walker B, et al, editors. HIV molecular immunology. Los Alamos, NM: Los Alamos National Laboratory: Theoretical Biology and Biophysics; 2009. [Google Scholar]
- 32.Ritchie AJ, Campion SL, Kopycinski J, et al. Differences in HIV-specific T cell responses between HIV-exposed and -unexposed HIV-seronegative individuals. J Virol. 2011;85:3507–16. doi: 10.1128/JVI.02444-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson M, Fonteneau JF, Lirvall M, Haslett P, Lifson JD, Bhardwaj N. Activation of HIV-1 specific CD4 and CD8T cells by human dendritic cells: roles for cross-presentation and non-infectious HIV-1 virus. AIDS. 2002;16:1319–29. doi: 10.1097/00002030-200207050-00003. [DOI] [PubMed] [Google Scholar]
- 34.Biasin M, Piacentini L, Lo Caputo S, et al. TLR activation pathways in HIV-1-exposed seronegative individuals. J Immunol. 2010;184:2710–7. doi: 10.4049/jimmunol.0902463. [DOI] [PubMed] [Google Scholar]
- 35.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misse D, Yssel H, Trabattoni D, et al. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J Immunol. 2007;178:407–15. doi: 10.4049/jimmunol.178.1.407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.