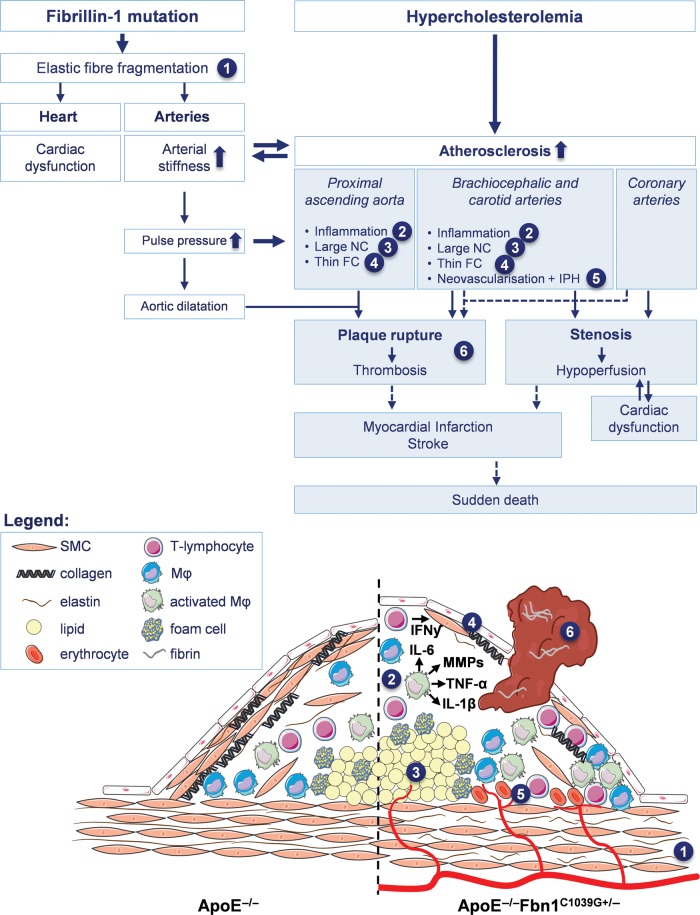
Figure 6.
Schematic overview of mechanisms leading to the formation of vulnerable plaques, plaque rupture, myocardial infarction, stroke, and sudden death in ApoE−/−Fbn1C1039G+/− mice (white boxes indicate the effects of each genotype separately, blue boxes the combination of both). The C1039G+/− mutation in the fibrillin-1 gene causes elastic fibre fragmentation (1), resulting in both increased arterial stiffness (and consequently elevated pulse pressure and progressive aortic dilatation) and cardiac dysfunction (due to the decreased contractility of the cardiomyocytes). In hypercholesterolemical ApoE−/−Fbn1C1039G+/− mice, this elastin fragmentation and arterial stiffness lead to the development of large plaques with a highly unstable phenotype, characterized by enhanced inflammation (2) large necrotic cores (NC) (3) and a thin fibrous cap (FC) (4). Additionally, in the brachiocephalic and carotid arteries intraplaque neovascularisation and haemorrhage (IPH) are abundantly present (5). Due to the elevated pulse pressure and extensive aortic dilatation (especially in the ascending aorta), the mechanical stress on plaques is increased, leading to rupture and subsequent thrombus formation (6). The pronounced atherosclerosis also leads to increased carotid and coronary artery stenosis, resulting in cerebral and/or cardiac hypoperfusion. Since ApoE−/−Fbn1C1039G+/− mice are already sensible to develop cardiac dysfunction as a consequence of the genotype, decreased cardiac perfusion may further impair cardiac function. Plaque rupture with (occlusive) thrombosis as well as hypoperfusion of the heart and brain most likely result in myocardial infarction, stroke, and eventually sudden death.