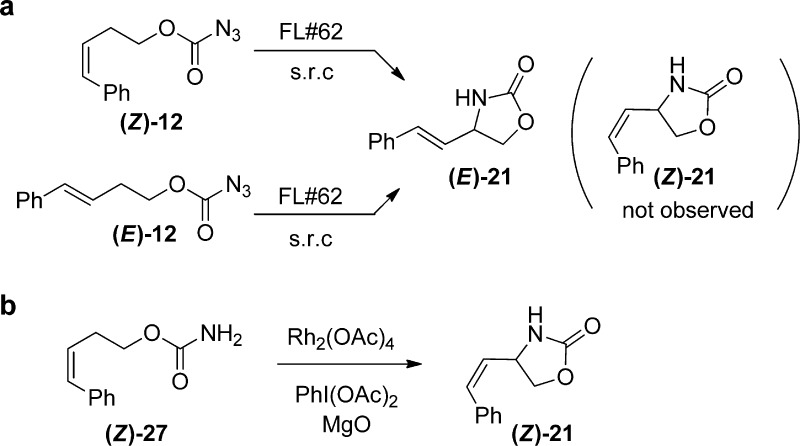
Figure 3.
Rearrangement studies. (a) FL#62-catalyzed cyclization of (E)-12 (or its isomer (Z)-12) leads to formation of trans oxazolidinone (E)-21, as determined by HPLC (Figure S3 in SI) and GC-MS analysis. The Z → E rearrangement in case of (Z)-12 is indicative of the formation radical intermediate via HAA. (b) Rhodium-catalyzed cyclization of carbamate Z-27 is not accompanied by scrambling of the double bond configuration, in agreement with the concerted nitrene C–H insertion mechanism proposed for these catalysts.51