Abstract
Clinical microbiology testing is crucial for the diagnosis and treatment of community and hospital-acquired infections. Laboratory scientists need to utilize technical and problem-solving skills to select from a wide array of microbial identification techniques. The inquiry-driven laboratory training required to prepare microbiology graduates for this professional environment can be difficult to replicate within undergraduate curricula, especially in courses that accommodate large student cohorts. We aimed to improve undergraduate scientific training by engaging hundreds of introductory microbiology students in an Authentic Large-Scale Undergraduate Research Experience (ALURE). The ALURE aimed to characterize the microorganisms that reside in the healthy human oral cavity—the oral microbiome—by analyzing hundreds of samples obtained from student volunteers within the course. Students were able to choose from selective and differential culture media, Gram-staining, microscopy, as well as polymerase chain reaction (PCR) and 16S rRNA gene sequencing techniques, in order to collect, analyze, and interpret novel data to determine the collective oral microbiome of the student cohort. Pre- and postsurvey analysis of student learning gains across two iterations of the course (2012–2013) revealed significantly higher student confidence in laboratory skills following the completion of the ALURE (p < 0.05 using the Mann-Whitney U-test). Learning objectives on effective scientific communication were also met through effective student performance in laboratory reports describing the research outcomes of the project. The integration of undergraduate research in clinical microbiology has the capacity to deliver authentic research experiences and improve scientific training for large cohorts of undergraduate students.
INTRODUCTION
Background
Clinical microbiology testing is an essential component of the social safety net, playing pivotal roles in healthcare, disease surveillance, food monitoring, and veterinary testing. However, decades of austerity measures have resulted in contraction of both the number of laboratories available and the selection pool of adequately trained laboratory technicians (22, 23). These understaffed laboratories cannot resource mentorship programs or high numbers of clinical apprenticeships (14), shifting the responsibility in training diagnostic technicians toward undergraduate science programs. Correspondingly, undergraduate curricula must adapt to the changing professional skill sets demanded of their graduates.
The American Society for Microbiology (ASM) established a concept-based curriculum for undergraduate microbiology that places a strong emphasis on learning activities that promote scientific thinking and critical-reasoning (20). This concept-based curriculum highlights the need to train students in selecting diagnostic tools, interpreting results through critical evaluation, then communicating the conclusions—all invaluable skills within the diagnostic laboratory. Research-based learning through inquiry-driven exercises has been shown to be an effective way to promote engagement and deep-learning through active problem solving (24), as well as deliver higher learning gains when compared with didactic instruction (19). Many programs have already adopted student-driven inquiry within the undergraduate laboratory, often directly integrating research projects designed to generate novel experimental data in the form of Authentic Large-Scale Undergraduate Research Experiences (ALUREs) (12, 19, 27). The learning objectives of these ALUREs are often directly aligned with the competencies across laboratory and research skills, and there has been a positive correlation between ALURE participation and student interest in scientific careers (12, 27).
This study describes an ALURE in mapping the human oral microbiome that adopts an inquiry-driven approach to learn about the wide suite of diagnostic tests available in microbial identification and the relative merits of culture-dependent versus culture-independent methods. Distinguishing the bacterial composition of healthy and diseased oral cavities is an important research project, as differential bacterial colonization has been implicated as a possible cause of dental caries and periodontitis (2, 4), as well as systemic issues including cardiovascular disease and diabetes (reviewed in (9)). Identification of oral microorganisms through culture-dependent methods results in selection bias toward cultivable organisms, and recent studies have utilized 16S rRNA gene amplicon sequencing to circumvent this bias (1, 3, 15). Correspondingly, due to its polymicrobial nature, the human oral cavity can generate clinical samples that clearly highlight the differences in diagnostic resolution across culture-dependent and culture-independent microbial identification techniques and allow hundreds of students to collectively generate their own microbiome data. This ALURE aims to engage the students in research activities on their own oral swabs while providing hands-on experience in technical laboratory skills, critical reasoning, and problem-solving skills within diagnostic microbiology.
Intended audience
The curriculum activity described within this article uses molecular biology, next-generation sequencing technology, and clinical diagnostic protocols and is intended for undergraduate students pursuing Microbiology, Biology, or Biotechnology majors. The activity is targeted at an introductory microbiology course for second-year students within a four-year undergraduate science program. Courses with a large student cohort (in excess of 100 students) are ideal, as single sequencing runs can accommodate multiplexing of hundreds of 16S rRNA gene amplicon samples, providing statistical power for correlative analyses.
Learning time
This activity was conducted in four three-hour laboratory sessions, with one week separating each session to allow for sufficient time to process individual samples for incubation and sequencing. Assuming all prerequisite student knowledge is met, the activity can be implemented at the beginning of the semester within a course’s learning sequence. An overview of the activity’s week-by-week outline as well as preparation time required is provided in Table 1.
TABLE 1.
Overview of oral microbiome module.
| Session | Activity Outline | Preparation Required |
|---|---|---|
| Week 1: Core skill building | Hands-on introduction to light microscopy, Gram-staining, and aseptic culturing techniques. | 1–2 days (depending on class size) to prepare agar plates, culture media, and demonstration microorganisms for students to work on. Incubation of inoculated samples at 37°C for 24 hours and storage at 4°C until session 2. |
| Week 2: Sampling the human oral microbiome | Conduct mouth-swabs for bacterial DNA extraction (for PCR and 16S rRNA sequencing) and inoculation of selective and differential culture media (blood agar, mannitol salt agar, and mitis salivarius agar plates). | 2 weeks required to optimize polymerase chain reaction (PCR) amplification of mouth swab DNA, sequencing of DNA, and clustering reads into operational taxonomic units (OTU). Gel electrophoresis images and OTU tables need to be ready for students by session 4. Incubation of inoculated samples at 37°C for 24 hours and storage at 4°C until session 3. |
| Week 3: Culture-dependent identification of oral microbes | Presumptive identification of oral microbiota from culture-based diagnostic tests, including Gram-staining, colony growth on selective and differential agar media, biochemical testing, and immunological testing. | 1–2 days (depending on class size) to prepare demonstration microorganisms, biochemical and immunological testing kits. |
| Week 4: Culture-independent identification of oral microbes | Analysis and interpretation of data collated across culture-dependent and culture-independent identification of oral microbiome across student cohort. | 1–2 days to collate gel electrophoresis images and OTU tables for all students within cohort. Microbiome data can be presented on learning management systems online, and/or provided to students in class. |
Prerequisite student knowledge
As a prerequisite for this ALURE, students are required to complete an introductory first-year biology course covering basic cell theory, the central dogma of biology, bacterial cell structure, microbial growth, as well as metabolic and phylogenetic diversity. Previous laboratory experience in pipetting, light microscopy, and DNA gel electrophoresis is also expected. The molecular phylogeny principles behind DNA sequencing and 16S rRNA metagenomic analyses are not assumed knowledge, and they are covered in detail within hands-on learning activities in the ALURE laboratory classes.
Learning objectives
By the end of this ALURE module, students should be able to:
Safely handle, isolate, and culture microorganisms using aseptic technique;
Prepare solutions and reaction mixes through accurate calculations, measurements, and pipetting;
Design and plan experimental approaches to identify microorganisms using both culture-dependent and culture-independent methods;
Clearly communicate experimental results through the accurate recording and professional presentation of laboratory observations in text and graphical formats; and
Critically evaluate scientific findings through sourcing peer-reviewed literature.
PROCEDURE
Materials
Session 1. For the visualization of microorganisms, each student was provided with one light microscope fitted with ×10, ×40, and ×100 objectives (OLYMPUS) and one set of Gram-stained smears of Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Streptococcus pyogenes (S. pyogenes), and Bacillus subtilis (B. subtilis). All cultures were obtained from the Australian Collections of Microorganisms (www.amrin.org/CultureCollections.aspx). Four trypticase soy agar plates (TSA—5 g/L enzymatic digest of Casein, 5 g/L enzymatic digest of Soybean meal, 5 g/L NaCl, 15 g/L agar—to be stored at 4°C before use) inoculated with E. coli, S. aureus, S. pyogenes, and B. subtilis were also provided. TSA plates could alternatively be purchased from Becton Dickinson.
One Gram-staining kit (Becton Dickinson) containing Gram crystal violet (3 g/L crystal violet, 5% (v/v) isopropanol, 5% (v/v) ethanol/methanol), Gram iodine (3.3 g/L iodine crystals, 6.6 g/L potassium iodide), Gram decolorizer (25% (v/v) acetone, 75% (v/v) isopropanol), and Gram safranin (4 g/L safranin O powder, 20% (v/v) ethanol/methanol) was provided per student. The Gram-staining kit could be stored at room temperature. One Bunsen burner (Labtek), one wire loop, and a set of glass slides were also provided for each student.
For the development of competencies in aseptic technique, each student was provided with one TSA plate inoculate with E. coli, one 5-mL broth mixed culture of E. coli and Micrococcus luteus (M. luteus), one 10-mL sterile broth in McCartney bottle, one tube of sterile peptone water (Becton Dickinson, 10 g/L peptone, 5 g/L NaCl—to be stored at 4°C before use), one 10-mL sterile distilled water for moistening swabs, one sterile plugged test tube, one sterile 10-mL graduated pipette, one sterile Pasteur pipette, one sterile swab (Becton Dickinson), three sterile TSA plates and incubation containers and racks for 28°C and 37°C.
Session 2. In order to extract genomic DNA from mouth swabs, each student was provided with micropipettors and accompanying pipette tips, and a BUCCALAMP DNA Extraction Kit (to be stored at 4°C before use) with MASTERAMP Brush Soft Pack (Epicentre). For the ensuing polymerase chain reaction (PCR), 16S rRNA genes were PCR amplified from the extracted DNA using broad-specificity primers (Sigma-Aldrich): 803F (803Fa-5′:TTAGATACCCTGGTAGTC; 803Fb-55′:TTAGATACCC SGGTAGTC; 803Fc-55′:TTAGATACCCYHGTAGTC; 803Fd-55′:TTAGAGACCCYGGTAGTC; mixed at ratios of 2a:b:c:d), and 1392wR (ACGGGCGGTGWGTRC). 10× buffer, MgCl2, BSA, dNTPs, and Taq DNA polymerase (Fisher Scientific) were also provided (to be stored at 4°C before use).
For gel electrophoresis analysis, the PCRs were mixed with loading dye (Fermentas) before running out at 120 V for 30 minutes in 0.8% agarose gels. Expected band sizes of PCRs were compared against standard bands from 100 bp DNA ladder (New England Biolabs).
To inoculate agar plates using their mouth swabs, each student was provided with three sterile swabs to inoculate a blood agar plate (15 g/L enzymatic digest of Casein, 5 g/L enzymatic digest of soybean meal, 5 g/L NaCl, 15 g/L agar, 5% sterile defibrinated blood—to be stored at 4°C before use), a mannitol salt agar plate (5 g/L enzymatic digest of casein, 5 g/L enzymatic digest of animal tissue, 1 g/L beef extract, 10 g/L D-mannitol, 75 g/L NaCl, 0.025 g/L phenol red, 15g/L agar—to be stored at 4°C before use) and a mitis salivarius agar plate (15 g/L enzymatic digest of casein, 5 g/L enzymatic digest of animal tissue, 50 g/L sucrose, 1 g/L dextrose, 4 g/L K2HPO4, 0.075 g/L trypan blue, 0.0008 g/L crystal violet, 15 g/L agar—to be stored at 4°C before use). Blood agar, mannitol salt agar, and mitis salivarius agar plates could alternatively be purchased from Becton Dickinson.
Session 3. In order to identify microbes from their own mouth swabs, each student was provided with a Gram-staining kit, Bunsen burner, wire loop, glass slides, and light microscope as per session 1. In addition to this, one set of demonstration blood and mannitol salt agar plates inoculated with S. aureus, Staphylococcus epidermidis (S. epidermidis), Staphylococcus saprophyticus (S. saprophyticus), and M. luteus were provided for Staphylococcal identification. For Streptococcal identification, each student was also provided with a set of demonstration blood agar and mitis salivarius agar plates inoculated with S. pyogenes, Streptococcus agalactiae (S. agalactiae), Streptococcus mitis (S. mitis), Enterococcus faecalis (E. faecalis), and Streptococcus pneumonia (S. pneumonia).
Each student was provided with one of novobiocin, bacitracin, and optochin antibiotic discs (Becton Dickinson—to be stored at 4°C before use), as well as 5 mL of 10% H2O2 for the standard operating protocols used in identifying Staphylococcus and Streptococcus species. One each Slidex Strepo plus and Slidex Staph plus latex agglutination testing kits (Biomerieux—to be stored at 4°C before use) was also provided per student.
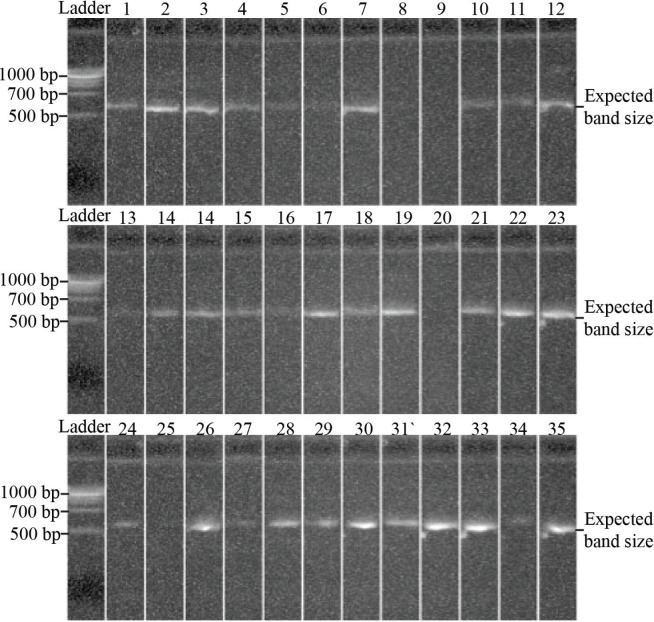
Session 4. To generate the 16S rRNA sequencing outputs, amplicons were sequenced using the Roche 454 GS-FLX Titanium platform, with filtering and error correction using the Acacia software (7) and the QIIME analysis pipeline (8). These data were then clustered into 97% identity operational taxonomic units (OTUs) (18), and classified using the Greengenes reference database (10). Select publications on the bioinformatics analysis were provided to students as preparation material and background reading. Within the session, the bacteria identified by culture-dependent techniques, PCR gel results from session 2, and OTU tables from 16S rRNA sequencing were provided for all mouth swabs collected across the student cohort. Explicit instructions were provided regarding how to interpret gel electrophoresis and OTU table data (sample gel images and OTU tables are provided in Fig. 2 and Table 4; instructions provided in Appendix 1). Students were encouraged to discuss key trends across the microbiome data and possible graphical/tabular formats of presentation to convey these observations.
FIGURE 2.
Sample student gel electrophoresis results. Polymerase chain reactions (PCRs) from student oral samples were mixed with loading dye (Fermentas) before running out at 120 V for 30 minutes in 0.8% agarose gels. Expected PCR band sizes were compared against standard bands from 100 bp DNA ladder (New England Biolabs). A subset of the total samples collected is shown.
TABLE 4.
Sample operational taxonomic unit (OTU) table for oral microbiome data across unique sample IDs. from de-identified student volunteers.
| Taxon |
Unique Sample IDs
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Actinomyces | 0.016 | 0.02 | 0.063 | 0.026 | 0.029 |
| Corynebacterium | 0.002 | 0.012 | 0.003 | 0 | 0.065 |
| Rothia | 0.052 | 0.004 | 0.015 | 0.022 | 0.052 |
| Propionibacterium | 0 | 0.001 | 0 | 0.001 | 0 |
| Atopobium | 0.002 | 0.003 | 0.019 | 0 | 0.001 |
| Family Porphyromonadaceae | 0.001 | 0.003 | 0 | 0 | 0 |
| Porphyromonas | 0.001 | 0.018 | 0.009 | 0.012 | 0.019 |
| Tannerella | 0 | 0.003 | 0.002 | 0.001 | 0 |
| Prevotella | 0.024 | 0.208 | 0.179 | 0.041 | 0.108 |
| Family Flavobacteriaceae | 0 | 0.003 | 0.002 | 0.002 | 0.017 |
| Capnocytophaga | 0.011 | 0.054 | 0.004 | 0.036 | 0.07 |
| Gemella | 0.008 | 0.026 | 0.012 | 0.056 | 0.021 |
| Abiotrophia | 0 | 0 | 0.001 | 0.002 | 0.001 |
| Granulicatella | 0.01 | 0.003 | 0.007 | 0.025 | 0.011 |
| Lactobacillus | 0 | 0 | 0 | 0 | 0.002 |
| Streptococcus | 0.059 | 0.092 | 0.068 | 0.189 | 0.102 |
| Order Clostridiales | 0 | 0.001 | 0.015 | 0.001 | 0 |
| Family Clostridiales Family XI Incertae Sedis | 0 | 0.004 | 0 | 0 | 0 |
| Eubacterium | 0.001 | 0.003 | 0.018 | 0 | 0.001 |
| Family Lachnospiraceae | 0.002 | 0.022 | 0.007 | 0.002 | 0.004 |
| Moryella | 0.002 | 0.003 | 0.006 | 0.002 | 0.002 |
| Oribacterium | 0.004 | 0.009 | 0.021 | 0.002 | 0.012 |
| Family Veillonellaceae | 0 | 0.003 | 0.007 | 0.001 | 0 |
| Dialister | 0.001 | 0.001 | 0 | 0 | 0.001 |
| Selenomonas | 0.007 | 0.021 | 0.017 | 0.004 | 0.007 |
| Veillonella | 0.033 | 0.024 | 0.162 | 0.091 | 0.028 |
| Fusobacterium | 0.081 | 0.258 | 0.054 | 0.012 | 0.05 |
| Leptotrichia | 0.004 | 0.029 | 0.01 | 0.001 | 0.02 |
| Lautropia | 0.001 | 0.001 | 0.004 | 0 | 0.023 |
| Hylemonella | 0 | 0 | 0 | 0 | 0.001 |
| Family Neisseriaceae | 0.067 | 0.046 | 0.088 | 0.095 | 0.24 |
| Campylobacter | 0.015 | 0.015 | 0.051 | 0.011 | 0.012 |
| Cardiobacterium | 0.001 | 0.005 | 0 | 0.003 | 0.006 |
| Actinobacillus | 0.001 | 0.001 | 0.005 | 0.249 | 0.001 |
| Aggregatibacter | 0 | 0.001 | 0.001 | 0 | 0.009 |
| Haemophilus | 0.577 | 0.073 | 0.098 | 0.099 | 0.061 |
| Moraxella | 0 | 0 | 0 | 0 | 0 |
| Pseudomonas | 0.004 | 0.005 | 0.004 | 0.001 | 0.001 |
| Phylum SR1 | 0 | 0.002 | 0.002 | 0 | 0 |
| Treponema | 0 | 0 | 0.003 | 0 | 0 |
| Order EW055 | 0.001 | 0.002 | 0.019 | 0 | 0.002 |
Relative abundance (0 to 1) is presented alongside all taxa detected across all oral samples. A subset of the total samples collected is shown.
Student instructions
The laboratory manual provided as student instructions is attached as Appendix 1. Students were provided with the laboratory manual at the start of the semester, at least one week prior to the commencement of the ALURE module. Apart from group-discussion activities embedded throughout the module, the laboratory tasks are designed for individual students to perform. The activities are also readily adaptable for student pairs within large class sizes, with one out of every two students providing an oral swab for culturing and sequencing. In these cases, each student can still develop competencies for the standard operating protocols, albeit potentially on demonstration plates instead of their own oral sample.
Faculty instructions
The oral microbiome ALURE can be run over four three-hour sessions, spanning across four weeks (an overview of which is presented in Table 1). At the conclusion of each session, cultures are incubated overnight and stored at 4°C until the following session for students to examine. To accommodate large class sizes, teaching assistants can be allocated groups of 10–12 students to supervise throughout the module, demonstrating techniques, monitoring progress, and facilitating group discussions. Detailed instructions for each of the four sessions are provided in Appendix 2.
Suggestions for determining student learning
The oral microbiome sampling project is an inquiry-driven research experience, and an essential component of authentic research involves the communication of scientific findings in a format consistent with professional scientific standards. Accordingly, the assessment task for this project revolved around an individual laboratory report following the structural conventions of a scientific publication, requiring students to outline the background of the discipline, the aims and hypotheses of the study, a summary of the methods utilized, clearly presented results in both text and graphical forms, and a discussion section interpreting the validity and significance of the findings while referring to relevant peer-reviewed literature. The report was limited to 800 words, not including figures, tables, legends, and bibliography, and student submissions were monitored for plagiarism via Turnitin.
In order to effectively complete the laboratory activities across the four sessions, students needed to be able to identify and explain how the structural components and physiological diversity of microbial cells allow scientists to perform taxonomic identification of microorganisms. Students also needed to competently utilize core laboratory techniques such as microscopy and selective culturing of microorganisms to identify specific oral microbial species, while contrasting these culture-dependent diagnostic results with 16S rRNA gene sequencing within the report. The individual laboratory report assessment task then required students to clearly communicate and present their scientific findings within a written format, and undertake critical evaluation of experimental validity by referencing peer-reviewed sources. The integration of this assessment task with the learning activities in this project directly align with all five of the module’s learning objectives, which is consistent with Biggs’ theory of constructive alignment in course design (5).
The marking rubric for the laboratory report spanned numerous criteria including presentation, knowledge of background theory, effective introduction of project aims and hypotheses, clear presentation of results, explanation and interpretation of trends, and use of sources for critical evaluation (Table 2). The criteria covered the explanation, presentation, interpretation, and critical evaluation of experimental results, as this directly aligned with learning objectives 4 and 5 for this module, revolving around the clear communication and critical evaluation of scientific findings. The laboratory report is the primary mode of assessment for the learning objectives of this ALURE module, and it is through the lens of this task in the 2012 and 2013 offerings that sample student data will be outlined in the following section. This does not exclude the use of alternative assessment tasks (e.g. oral presentations, examinations) for other materials and content within the course overall.
TABLE 2.
Laboratory report marking rubric.
| Criteria | Fail | Pass | High Pass |
|---|---|---|---|
| Presentation (1 mark) | Grammar and spelling errors throughout AND Inconsistent visual layout lacking clarity 0 marks |
Minor grammar and spelling errors OR Inconsistent communication and consistent visual layout 0.5 marks |
Accurate grammar and spelling AND Clear communication and consistent visual layout 1 mark |
| Knowledge of background theory (2 marks) (Q2+5) | Flawed AND inaccurate description of background theory throughout report 0–0.5 marks |
Flawed OR inaccurate description of background theory throughout report 1 mark |
Accurate and thorough description of background theory throughout report 1.5–2 marks |
| Introduction, Aims, Hypothesis (1 mark) | Incomplete AND inaccurate description of aims and hypotheses for project 0 marks |
Incomplete OR inaccurate description of aims and hypotheses for project 0.5 marks |
Accurate and complete description of specific project aims and hypotheses 1 mark |
| Clear recording of observations and presentation of results (3 marks) | Incomplete explanation of results in text AND Inaccurate presentation of figures/tables AND Incomplete figure legends 0–1 mark |
Incomplete explanation of results in text OR Inaccurate presentation of figures/tables OR Incomplete figure legends 1.5–2 marks |
Clear explanation of results in text AND Clear presentation of figures/tables AND Detailed and complete figure legends 2.5–3 marks |
| Explanation and interpretation of observed trends (1 mark) | Incomplete AND inaccurate summary of results and conclusions 0 marks |
Incomplete OR inaccurate summary of results and conclusions 0.5 marks |
Clear and concise summary of results and conclusions 1 mark |
| Use of sources to critically evaluate findings (2 marks) | Inappropriate selection of sources AND Invalid/insufficient comparisons between sources and findings 0–0.5 marks |
Inappropriate selection of sources OR Invalid/insufficient comparisons between sources and findings 1 mark |
Appropriate selection of sources AND Valid and insightful comparisons between sources and findings 1.5–2 marks |
| TOTAL MARK OUT OF 10 | |||
The criteria within this marking rubric were used across all laboratory report submissions to assess student performance.
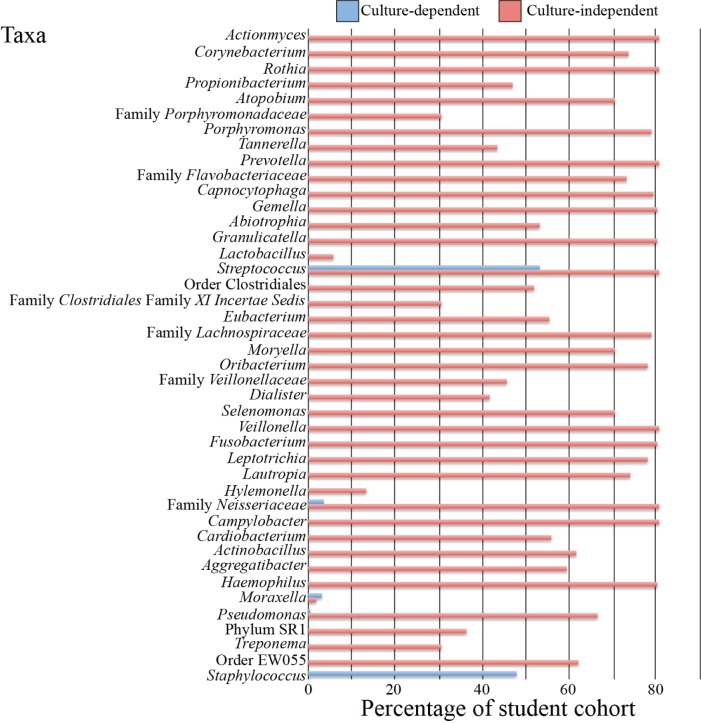
Sample data
Examples of student attempts to present qualitative and quantitative data are shown in Table 3 and Figure 1, where they were required to effectively summarize not only the microbial profile of their own oral cavity, but also across the entire student cohort as determined through culture-dependent and culture-independent testing. Students were expected to interpret OTU tables (Table 4) and gel electrophoresis results (Fig. 2) to determine the outcome of the PCR on oral samples obtained throughout the cohort, as well as the presence or absence of bacterial taxa within mouth swab samples.
TABLE 3.
Sample student data for culture-dependent identification of oral bacteria.
| Test | Result for Cultured Oral Swab Isolate |
|---|---|
| Colony characteristics | Yellow, β-hemolytic colony on blood agar plate; yellow colonies surrounded by bright yellow zones on mannitol salt agar plate |
| Gram-stain result | Gram-positive cocci in grape-like clusters |
| Catalase test | Appearance of bubbles when colony treated with H2O2 – Catalase positive |
| Coagulase test | No clumping when treated with latex beads coated with antibodies targeting Staphylococcal coagulase – Coagulase negative |
| Novobiocin sensitivity | Inhibition of bacterial growth surrounding antibiotic disc (annular radius of zone of inhibition > 6 mm) – Novobiocin sensitive |
| Presumptive identification | Staphylococcus epidermidis |
Student oral swabs were inoculated onto blood agar, mannitol-salt agar, and mitis salivarius agar plates and incubated at 37°C overnight. The resultant colonies were characterized by Gram-staining, biochemical and immunological testing, and antibiotic sensitivity.
FIGURE 1.
Sample student data for identification of oral bacteria via culture-dependent and culture-independent techniques. Culture-dependent identification of oral swabs was carried out as previously described in Appendix 1. Culture-independent identification involved oral swabbing and genomic DNA extraction of student participants using an Epicentre DNA extraction kit. This was followed by polymerase chain reaction (PCR) amplification of the V5–V8 portions of the 16S rRNA gene, which was then sequenced for bacterial identification.
With respect to student performance in explaining, interpreting, and critically evaluating experimental results, sample submissions that received Fail, Pass, or High Pass grades are displayed in Tables 5 and 6. Common student mistakes in these criteria included a lack of in-depth description of the taxa identified by both culture-dependent and culture-independent techniques and failing to make meaningful comparisons between significant findings in recent peer-reviewed research articles and their own experimental data. High-performing students were able to extrapolate metabolic and physiological similarities across the bacterial taxa identified by various diagnostic techniques, in an attempt to rationalize the discrepancies in culture-dependent and culture-independent identification of oral microorganisms. The quality of the critical evaluation of experimental results in relation to peer-reviewed findings directly aligned with learning objectives 4 and 5.
TABLE 5.
Sample student responses in explaining and interpreting observed trends.
| Grade | Sample Responses |
|---|---|
| Fail |
Student text: “Culture independent methods identified many more bacterial species than culture dependent methods did. Culture independent methods identified 41 different genera while culture dependent methods only identified 5 different genera. All of the bacteria identified by culture dependent methods were identified by culture dependent methods except for Staphylococcus. It can be concluded that culture independent methods are able to identify more types of bacteria that are found in the mouth.” Justification: Missing in-depth description of taxa identified by culture-dependent/independent methods; failed to highlight potential similarities amongst taxa identified; did not display correct understanding of difference between identification methods. |
| Pass |
Student text: “Although some taxa, such as Streptococcus, were present in 100% of the 16S rRNA results, this was not the case with culture-dependent results. This could be due to the fact that the culture-dependent testing was performed by hundreds of students. Mistakes were probable, and any false negative results would have been included. This is as opposed to sequencing where a poor sample collection would produce minimal DNA and therefore be excluded from the results. Also, no obligate anaerobes were present in the culture-dependent results. Facultative anaerobes were present, so perhaps these out competed the obligate anaerobes. It is also possible that the obligate anaerobes collected were killed due to prolonged O2 exposure during collection and plating.” Justification: Missing in-depth description of taxa identified by culture-dependent/independent methods; attempt made to highlight similarities amongst taxa identified, but not in sufficient detail; potential sampling errors were identified, albeit incompletely. |
| High Pass |
Student text: “A plethora of diverse bacteria were identified by the 16S rRNA sequencing and culture-dependent assays. Through 16S rRNA sequencing, many Gram-negative, anaerobic bacteria, such as Dialister, Selenomonas, and Veillonella were identified (Doan 2000, Sutter 1984). However, the most abundant bacteria (detected in greater than 99% of the culture-independent samples) were predominantly aerobic or facultative anaerobes (Drancourt 2004). Altogether, these abundant bacteria consisted of: Actinomyces, Prevotella, Gemella, Rothia, Granulicatella, Veillonella, Fusobacterium, Streptococcus, Family Neisseriaceae, Campylobacter and Haemophilus. The least abundant bacteria were Lactobacillus, Hylemonella, and Moraxella. The culture-dependent method did not identify the same diversity of microorganisms compared to 16S rRNA sequencing; all anaerobic bacteria identified by 16S rRNA were undetectable by the culture-dependent method. Also, Staphylococcus was not identified by 16S rRNA sequencing, however was detected in 48% of the participants through the culture-dependent method. This may be attributed to the antimicrobial properties of saliva that inhibit growth of certain bacteria, (MacFarlane 1974) and possible contamination by Staphylococcus from skin flora in the culture-dependent assay.” Justification: In-depth description of taxa identified by culture-dependent/independent methods; correlation of similarities in metabolic requirements of taxa detected; potential disparities in results explained using literature citations. |
TABLE 6.
Sample student responses in using sources to critically evaluate results.
| Grade | Sample Responses |
|---|---|
| Fail |
Student text: “Aas et al. (2005) attempted to define the normal oral bacterial flora (1), and the only similar bacteria found were of the Streptococcus group while others were not found within our techniques. Our list may be different from the article due to the different sampling method as well as the health of sample provider.” Justification: Incorrect comparison between cited study and own experiments; failure to describe differences in taxa detected in detail; omission of methodology employed in cited study (sample size, culture-dependent/independent protocols, demographics of volunteers). |
| Pass |
Student text: “Aas et al. (2005) performed a similar experiment to the present one (1). They reported that species from the genera Gemella, Granulicatella, and Streptococcus were present on the dorsal tongue of the majority of subjects. These results are in accord with data from the present study, as species from these genera were found in greater than 80% of subjects here. Species from the genera Gemella, Granulicatella, and Streptococcus were found in more than 80% of subjects’ oral cavities in our culture-independent tests. However, only Streptococcus was positively identified using culture-dependent methods. Aas et al. (2005) used 16S rRNA sequencing in their experiment, making their methods quite similar to the process used by us in the culture-independent part of our study. Therefore, it is expected that these results would be more comparable than our culture-dependent data and Aas et al.’s data.” Justification: Valid and sufficiently detailed comparison made between cited study and own experiments, both in taxa detected and identification protocols; omission of sample size, demographic data of volunteers. |
| High Pass |
Student text: “Research by Keijser and colleagues (2008) aimed to determine the number of different phyla and abundance of oral microflora in healthy adults (16), similar to this experiment and other studies (15). The vast majority of sequences (99.6%) belonged to one of seven phyla, all of which are common gastrointestinal flora with varying metabolic functions: Actinobacteria, Bacteroides, Firmicutes, Fusobacteria, Proteobacteria, Spirochetes and Phylum TM7. This is similar to results from this experiment. At a genus level Streptococcus, Veillonella, Prevotella, Corynebacterium and Actinomyces were identified as the predominant genera in saliva (16). This experiment found support for the seven main phyla and main genera suggested by previous research. Staphylococcus was found in culture-dependent but not in culture-independent techniques in this experiment; however, this was not supported in the literature. This could suggest that it should not be present, as in culture-independent methods. In this experiment a larger sample size was employed; however, data could vary due to individual differences such as socio-economic status, age, geolocation and health status rather than sample size. Antibiotics may interfere with the number of bacteria found in both techniques, which may impact this experiment (15). Culture-dependent methods may have been restricted due to lack of essential nutrients and growth factors from media, production of inhibitory substance by other bacteria and metabolic dependence on other species for growth (25).” Justification: Valid and sufficiently detailed comparison made between cited study and own experiments, both in taxa detected and identification protocols; in-depth discussion of sample population demographic factors that may influence experimental outcomes. |
Safety issues
The oral microbiome project involves sampling the oral cavities of hundreds of students who vary in age, ethnicity, and medical history; in light of this, there is a possibility of isolating pathogenic microorganisms. To safeguard against this, prior to the commencement of the ALURE, students are required to complete Biosafety Level 2 (BSL-2) safety training. This training covers the necessary personal protective equipment (PPE), the safe handling of microorganisms, and the disposal of biological waste in accordance with BSL-2 regulations. All students needed to be up to date for tetanus immunization. Students with health conditions such as pregnancy, allergies, or immune-compromised status should not directly handle or come into contact with cultured microorganisms.
The cultured microorganisms provided within the ALURE all belong to BSL-2 (moderate potential hazard), and all microbiological samples were treated as potential pathogens. Pipetting by mouth was strictly prohibited, and students were instructed on how to prevent splashing or aerosolizing bacterial colonies by allowing the flame-sterilized inoculation loop to cool prior to making contact with cultures. Enclosed protective footwear where both the upper foot and heel were covered, clean laboratory coats, safety glasses, and gloves were worn at all times within the laboratory to minimize direct contact with any microorganisms. Students were only permitted to handle their own oral swabs before processing for culture-dependent and culture-independent testing. Laboratory bench surfaces and objects such as pens and notebooks were decontaminated with 70% ethanol before and after each session, along with rigorous hand washing using detergents with residual antibacterial activity (e.g. 4% chlorhexidine). The use of touch-screen devices such as mobile phones and tablets was banned within the laboratory to minimize the possibility of surface contamination. All waste was disposed of in accordance with BSL-2 regulations, including the categorization of biological, chemical, and sharps waste into separate leak and puncture-resistant containers before decontamination via autoclaving.
DISCUSSION
Field testing
The oral microbiome ALURE was implemented within the 2012 and 2013 offerings of an introductory microbiology course at The University of Queensland (UQ), and the project protocols were cleared in accordance with the ethical review processes of UQ and with the guidelines of the National Statement on Ethical Conduct in Human Research 2007 in Australia (“DNA Sequencing of Microbial Populations” – Project Number 2012000755). Student participants provided their informed consent with regard to the publication and analysis of their de-identified microbiome results, performance in course assessment tasks, and responses to course-specific surveys. The survey questions evaluated student confidence in scientific skills, and survey responses were quantified using a 5-point learning-gains scale (0 = do not know how to do; 1 = not competent; 2 = need practice; 3 = competent; 4 = highly competent).
Evidence of student learning
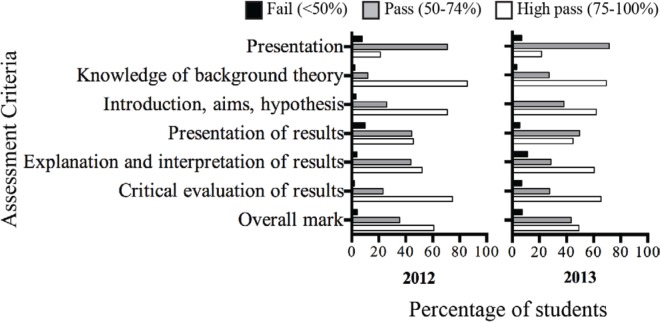
Student performance in the individual laboratory report is the primary indicator of learning gains resulting from the oral microbiome project. The percentage of students who received Fail, Pass, and High Pass grades in the report’s assessment criteria is displayed in Figure 3. Over 90% of the students received a Pass or a High Pass overall, with a less than 8% failure rate in both 2012 and 2013. The failure rate for each individual criterion was approximately 10% or lower in every instance across both offerings of the ALURE, suggesting that most students were able to adequately apply the core laboratory and communication competencies required to complete the laboratory report. Furthermore, a majority of students received a High Pass for the criteria relating to explanation and interpretation of results and using sources for critical evaluation in both 2012 and 2013, which represent core competencies that emphasize scientific thinking and deeper conceptual understanding and align with learning objectives 4 and 5 (20).
FIGURE 3.
Student performance across project report criteria (as per Table 1) in 2012 and 2013. The proportion of students within the course cohorts who achieved a Fail (<49% – black), Pass (50–74% – grey), or a High Pass (75–100% – white) within each of the criteria is depicted.
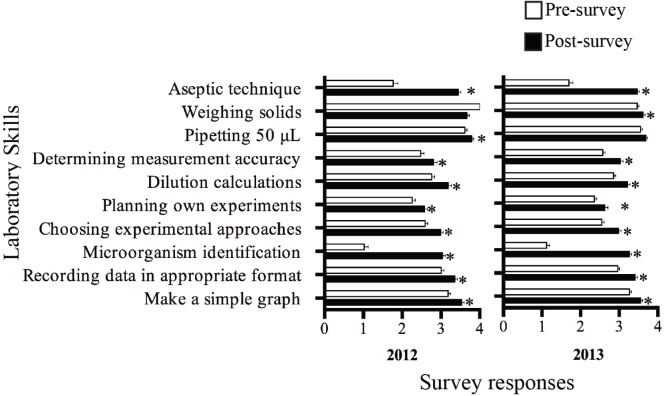
A strong component of discipline-specific research is the ability to competently perform fundamental scientific skills. To assess whether or not students improved in their mastery of laboratory skills following the ALURE, pre-and postsurvey responses to a 5-point confidence scale regarding fundamental scientific skills within the experimental laboratory were monitored. Statistically significant increases were reported in student confidence across a wide range of laboratory skills following the completion of the ALURE, including the ability to culture and maintain bacteria, identify pathogens using a variety of techniques, perform scientific calculations, and determine the accuracy of measurements (Fig. 4). This suggests that following the ALURE module, students perceive themselves as possessing increased competence in these skills, and their achieved learning gains align with learning objectives 1, 2, and 3. Increases in student confidence were also observed for the ability to make a simple graph to display experimental data, plan their own experiments, and record data in an appropriate format (Fig. 4), which were corroborated by their high performance in the corresponding project report criteria (Fig. 3). This combination of perception and performance data with regard to scientific communication and critical evaluation of results and peer-reviewed literature indicates achievement of student-learning gains that met learning objectives 4 and 5. These trends in student confidence and performance were observed across both the 2012 and 2013 offerings of the ALURE module, indicating the impact on student learning gains is reproducible in meeting the course’s learning objectives.
FIGURE 4.
Student perspectives on laboratory skill proficiency following the microbiome ALURE. Student confidence was measured through responses to survey questions in 2012 (n = 130) and 2013 (n = 197) on a 0–4 scale (0 = Do not know how to do; 1 = Not competent; 2 = Need practice; 3 = Competent; 4 = Highly competent). The mean survey response ± SEM is plotted above. * denotes a statistically significant difference between student survey responses before and after the laboratory experience as determined by the Mann-Whitney U-test (p < 0.05). ALURE = authentic large-scale undergraduate research experience; SEM = standard error of the mean.
Based on the collective student performance and perception data, it could be seen that the oral microbiome ALURE facilitated student development of fundamental research skills, meeting learning objectives 1–5 of the module. The positive impact of this ALURE on student development of research skills is consistent with previous reports in chemistry, biochemistry, and microbiology (12, 19, 26, 27), and this project has been able to provide another documented case of integrative research experiences that are adaptable for large undergraduate classes.
Possible modifications
The oral microbiome ALURE at UQ was implemented in a course of 300–400 students; the resources required to process sequencing runs for hundreds of clinical samples was made available through industry sponsorship (Roche). The project could be scaled down for smaller class sizes, although this would diminish the statistical power provided by a large sample population in correlating determinants of oral microbiota composition. A similar experimental approach could be adopted in mapping the microbiome across different parts of the human body, in line with the holistic approach adopted by the Human Microbiome Project (21) the culture-dependent tests and diagnostic standard operating procedures would need to be adjusted accordingly depending on common resident microbiota at the respective body sites.
If next-generation sequencing technology is not available for potential adopters, 16S rRNA amplicons can be ligated into plasmids before direct plasmid sequencing of the PCR fragments (17), which is feasible for smaller class sizes. Gel-based techniques are also an alternative to sequencing, with variations in DNA banding patterns used to discriminate between oral microbiome data across student samples. Existing protocols for pulse-field gel electrophoresis (PFGE) and multilocus sequence typing (MLST) for bacterial identification are well established (as reviewed in Ref. 13) and can be readily implemented.
The described activity also possesses the capacity to directly integrate bioinformatics exercises, where students could be provided with hands-on opportunities to familiarize themselves with bioinformatics software such as Acacia, the QIIME analysis pipeline, and reference databases used to generate the microbiome data. Students can learn to use these and other software packages by visualizing and measuring the degree of compositional similarity across bacteria taxa, as well as potentially incorporating elements of whole-genome sequencing and microbial genome annotation from previously described classroom exercises (6, 11).
SUPPLEMENTAL MATERIALS
Appendix 1: Student instructions for human oral microbiome ALURE
Appendix 2: Faculty instructions for human oral microbiome ALURE
ACKNOWLEDGMENTS
The authors thank the student cohort, Dr. Fiona May from the Australian Centre for Ecogenomics for performing the Roche 454 sequencing runs, and Dr. Kuo-Chang Lee and Igor Popovic for coordinating the laboratory sessions. This study was supported by a UQ Faculty of Science Teaching and Learning Grant 2012–2013, Roche Diagnostics Australia Pty Ltd, and internal funding provided by the School of Chemistry and Molecular Biosciences, The University of Queensland. The authors declare that there are no conflicts of interest.
Footnotes
Supplemental materials available at http://jmbe.asm.org
REFERENCES
- 1.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988–1994. J. Periodontology. 1999;70:13–29. doi: 10.1902/jop.1999.70.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Alcaraz LD, et al. Identifying a healthy oral microbiome through metagenomics. Clin Microbiol Infect. 2012;18(Suppl 4):54–57. doi: 10.1111/j.1469-0691.2012.03857.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker MR, et al. Molecular analysis of bacterial species associated with childhood caries. J Clin Microbiol. 2002;40:1001–1009. doi: 10.1128/JCM.40.3.1001-1009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggs J. Aligning teaching and assessing to course objectives Teaching and Learning in Higher Education: New Trends and Innovations (conference); University of Aveiro. 13–17 April 2003.2003. [Google Scholar]
- 6.Boyle MD. “Shovel-ready” sequences as a stimulus for the next generation of life scientists. J Microbiol Biol Educ. 2010;11:38–41. doi: 10.1128/jmbe.v11i1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bragg L, Stone G, Imelfort M, Hugenholtz P, Tyson GW. Fast, accurate error-correction of amplicon pyrosequences using Acacia. Nat Methods. 2012;9:425–426. doi: 10.1038/nmeth.1990. [DOI] [PubMed] [Google Scholar]
- 8.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontology. 2013;2000;62:271–286. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- 10.DeSantis TZ, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drew JC, Triplett EW. Whole genome sequencing in the undergraduate classroom: outcomes and lessons from a pilot course. J Microbiol Biol Educ. 2008;9:3–11. doi: 10.1128/jmbe.v9.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanauer DI, Jacobs-Sera D, Pedulla ML, Cresawn SG, Hendrix RW, Hatfull GF. Inquiry learning. Teaching scientific inquiry. Science. 2006;314:1880–1881. doi: 10.1126/science.1136796. [DOI] [PubMed] [Google Scholar]
- 13.Hyytia-Trees EK, Cooper K, Ribot EM, Gerner-Smidt P. Recent developments and future prospects in subtyping of foodborne bacterial pathogens. Future Microbiol. 2007;2:175–185. doi: 10.2217/17460913.2.2.175. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan RL, Burgess TE. The impending crisis. J Microbiol Biol Educ. 2010;11:140–143. doi: 10.1128/jmbe.v11i2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazor CE, et al. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J Clin Microbiol. 2003;41:558–563. doi: 10.1128/JCM.41.2.558-563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keijser BJ, et al. Pyrosequencing analysis of the oral microflora of healthy adults. J Dental Res. 2008;87:1016–1020. doi: 10.1177/154405910808701104. [DOI] [PubMed] [Google Scholar]
- 17.Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci USA. 1999;96:14547–14552. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunin V, Engelbrektson A, Ochman H, Hugenholtz P. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol. 2010;12:118–123. doi: 10.1111/j.1462-2920.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 19.Lord T, Orkwiszewski T. Moving from didactic to inquiry-based instruction in a science laboratory. Am Biol Teach. 2006;68:342–345. doi: 10.1662/0002-7685(2006)68[342:DTIIIA]2.0.CO;2. [DOI] [Google Scholar]
- 20.Merkel S. The development of curricular guidelines for introductory microbiology that focus on understanding. J Microbiol Biol Educ. 2012;13:32–38. doi: 10.1128/jmbe.v13i1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J. Metagenomic pyrosequencing and microbial identification. Clin Chem. 2009;55:856–866. doi: 10.1373/clinchem.2008.107565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahaley R. The changing roles of veterinary laboratories in Australia. Microbiol Australia. 2013;34:12–18. [Google Scholar]
- 23.Rogoski RR. Training tomorrow’s lab techs challenges today’s shrinking faculty. Med Lab Obs. 2010;42:30–31. [PubMed] [Google Scholar]
- 24.Schraw G, Flowerday T, Lehman S. Increasing situational interest in the classroom. Educ Psychol Rev. 2001;13:211–224. doi: 10.1023/A:1016619705184. [DOI] [Google Scholar]
- 25.Siqueira JF, Jr, Rocas IN. Exploiting molecular methods to explore endodontic infections: Part 1—current molecular technologies for microbiological diagnosis. J Endodontics. 2005;31:411–423. doi: 10.1097/01.don.0000157989.44949.26. [DOI] [PubMed] [Google Scholar]
- 26.Wang JTH, Schembri MA, Ramakrishna M, Sagulenko E, Fuerst JA. Immersing undergraduate students in the research experience. Biochem Mol Biol Educ. 2012;40:37–45. doi: 10.1002/bmb.20572. [DOI] [Google Scholar]
- 27.Weaver GC, Russell CB, Wink DJ. Inquiry-based and research-based laboratory pedagogies in undergraduate science. Nature Chem Biol. 2008;4:577–580. doi: 10.1038/nchembio1008-577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Student instructions for human oral microbiome ALURE
Appendix 2: Faculty instructions for human oral microbiome ALURE